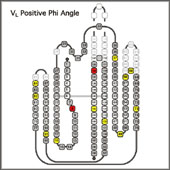
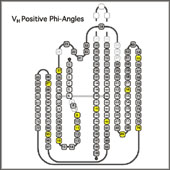
Variable Domain Torsion Angles
Immunoglobulin variable domains contain a number of positions with conserved positive Phi angles. Most, but not all of these positions show a strong preferences for Gly. Other residue types may have a destabilizing effect in those positions. VL kappa domains usually also contain two cis-prolines. cis-Pro L8 forms the "kink" in framework I. S.Spada et al. have shown that replacement of this cis-Pro by other residue types has a destabilizing effect. A similar cis-Pro is present in the equivalent position of many T-cell receptor variable domains. VL lambda and heavy chain variable domains have a one-residue deletion compared to VL kappa and do not contain conserved cis-prolines in the FR1 kink. A second conserved cis-Pro in Position L136 is responsible for the omega-loop conformation of the CDR 3 of most of the kappa domains
|
Things to come: Ramachandran plot by residue number
|