Antibody - Antigen Interactions
- The most important functional parameters of an Antibody (or of a Tcell receptor, for that matter) are the affinity and specificity of its interaction with the cognate ligand, the antigen or, for the TCR, the peptide/MHC complex. A careful analysis of the residues and types of interaction involved in antigen binding are of crucial importance not only in the engineering of individual antibodies (e.g. humanization by resurfacing or loop grafting), but even more so in the design of artifical antibody libraries randomized in CDR 1 and 2 as well as CDR 3. (A.Honegger & A.Plückthun, "Optimization of synthetic antibody libraries", in preparation)
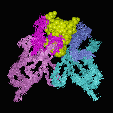
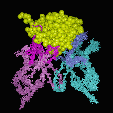
An analysis of the binding modes of haptens (small molecules), oligomers (peptides and oligosaccharides) and proteins shows that these prefer different binding modes: Haptens, having a limited total surface area, deeply embed themselfs into the VL/VH dimer interface. As a consequence, hapten binding antibodies frequently show a deep central cavity, long CDR L1 loops and a CDR H3 loop with an "open" conformation, allowing the hapten to bind as much as 80% of its total surface in the interaction. In contrast, proteins preferentially to a relatively flat binding surface: In a "closed" CDR H3 conformation, the CDR H3 loop packs down onto the central cavity, and the protein antigen binds on top of it. Short CDR L3 loops are preferred in protein binders.
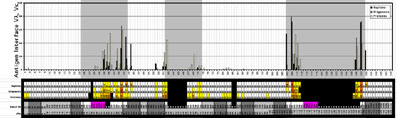
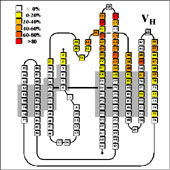
- Using the residue solvent accessible surface (rsa) macros provided on this website to analyse NACCESS result files, the antigen contact residues in the complexes could be identified by determining the relative differences in solvent accessible surface of each residue between the FV-fragment alone and the FV-Antigen complex.
|
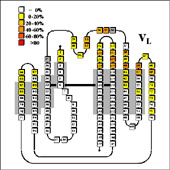
- From this, the average contribution of each VL residue and VH residue to the antigen interface could be determined for the three classes of complexes, and the average contact area could be calculated:
-
- Comparison of the sequence statistics revealed key positions which control access to the binding pocket. At the same time they reveal that many of the hapten contacts involve highly conserved residues, since the same residues are also involved in VL/VH dimer contacts.
|