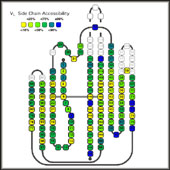
Side Chain Solvent AccessibilityThe program NACCESS (S. Hubbard and J. Thornton) was used to calculate the solvent accessibility of each residue in the isolated domains, FV fragments, Fab fragments and antigen complexes. The accessibility macros were used to collect the results into a common EXCEL worksheet and to convert the numeric values for the relative residue solvent accessibility into a color code: from yellow (dry) = strongly buried over shades of green to blue (wet)=fully solvent exposed. From the difference in accessible surface area between the isolated domains and the FV-fragments the residues contributing to the VL/VH dimer interface were identified. The differences between the rsa values of free FV-fragment and antigen-complexed FV fragment identified the residues contributing to the antigen contact interface, and the differences between FV fragment and Fab fragment the residues contributing to the V/C interfaces. For the representations shown here, the side chain accessibility was used, since they give the clearest picture, although the main-chain accessibility and the total residue accessibility were analyzed as well. |
Histogram of average side
chain solvent accessibility of each position:
Sequence alignment, color-coded for side chain accessibility of the individual domains: |
|
|
|||||||||||||||||
|
Last Modified by A.Honegger |
|||||||||||||||||