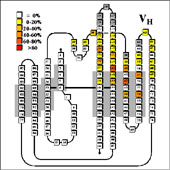
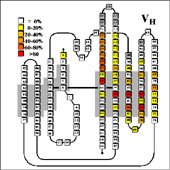
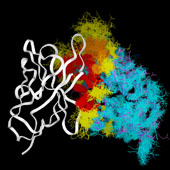
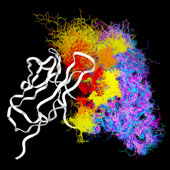
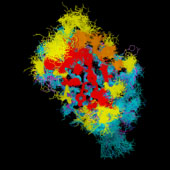
VL/VH Dimer Interface
- Given the combinatorial nature of antibody generation, the dimer interface residues have to be such that any VL domain can pair with any VH domain to form a functional interface. This places a heavy constraint on the conservation of the interface residues.
VL Dimer Contacts
|
VH Dimer Contacts
|
 |
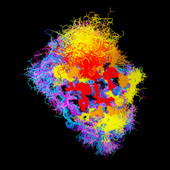 |
- At the same time, there is considerable overlap between the antigen interface and the dimer interface, particularely for hapten-binding antibodies, where the antigen frequently inserts into a deep binding pocket in the center of the barrel formed by the inner beta sheets of the two domains.
|
Dimer contact residues in individual domains are listed in the following pages:
|