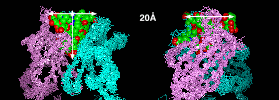Annotated sequence alignments of the VL and the VH domains of these antibodies shows which of the Fabs are related: Antigen contact analysis reveals which residues are actually involved in Antigen contacts |
Comparison of the different antibody-antigen complexes in the PDB database shows that the smaller the antigen molecule is, the deeper it has to bury in the antigen combining site to obtain a sufficient ly large interface area: Haptens usually insert into a deep binding pocket, while proteins show a relatively flat binding surface:
As this superposition of the catalytic antibody structures in the PDB shows, they are typical hapten complexes. The envelope of the superimposed ligands gives an idea of the maximal possible size of the binding pocket: A funnel-shaped cavity of about 20 Å diameter and a depth of about 20 Å, inserted along the axis of the VL/VH heterodimer. |
|
|
|||||||||||||||||
|
Last Modified by A.Honegger |
|||||||||||||||||