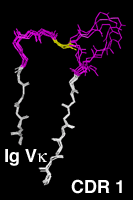
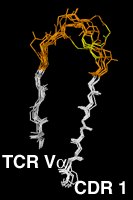
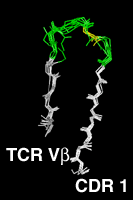
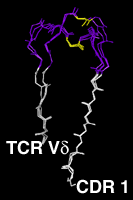
Residues 20 to 47The first complementarity determining region, CDR L1 and CDR H1 in antibodies is treated as a single loop in all existing numbering schemes. With CDR 1, the amino acid chain switches from the "outer" b-sheet to the "inner" one, which forms the heterodimer interface between the antibody light and heavy chain, between the alpha and beta chains in alpha-beta TCRs and between the gamma and delta chains in gamma-delta TCRs. Structurally, in the CDR 1 segment, one usually hydrophobic residue assumes a distinguished position. It intercalates between the two beta sheets and divides the CDR 1 into two loops . In our numbering scheme the residue number of this amino acid is 31. The outer, N-terminal loop is longest in some TCR Va domains and shortest in some Vl domains, which both show some length variability in this part of CDR 1. VH domains, as well as the majority of the Va domains, have a one-residue gap in position 28, Vk and Vb domains a two-residue gap in positions 27 and 28. CDR L1 of the kappa chain shows the largest degree of length variability, affecting only the loop C-terminal to the intercalating residue 31. Here the gap is centered on position 36. The highly conserved core tryptophan residue (Kabat H36, L35, A34, B34, C35 or D35, IMGT 41) is always numbered "43" in our scheme. Click on the pictures for an enlarged and annotated image |
The Complementarity Determining Region 1Since the two gap positions, centered on residues 28 and 36, are treated separately and the intercalated residue is always kept fixed at position 31, a shorter N-terminal loop cannot compensate a longer C-terminal loop. Compared to the IMGT scheme, 2 more positions are needed to represent the length variability, since in the IMGT alignment, an insertion in the N-terminal loop of CDR 1 can be compensated by a short C-terminal loop. |
|
|
|||||||||||||||||
|
Last Modified by A.Honegger |
|||||||||||||||||