 |
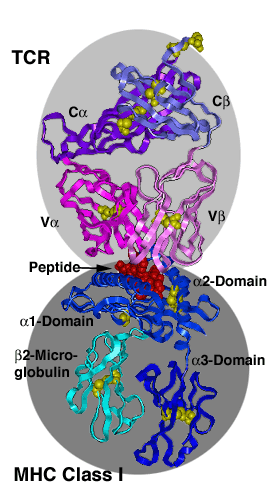
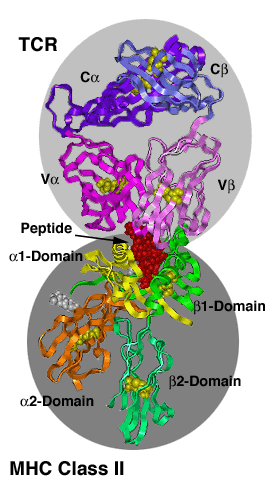
MHC-TCR ComplexesThe major histocompatibility complex (MHC) encodes two highly polymorphic cell surface molecules: MHC class I (left image) and MHC class II (right image), which present peptides for recognition by the T-cell receptors (TCR) on the surface of T-lymphocytes. The antigen recognition unit of the TCR ressembles a Fab fragment with a C-terminal transmembrane segment and a short cytoplasmic tail added to each chain. Each chain consists of a variable and a constant domain. The a and b chains fom the ab TCRs, g and d-chains the gd TCRs. The TCR recognition unit is associated with CD3, the signalling unit of the T-cell receptor, consisting of multiple chains, some of which also contain extracellular domains belonging to the immunoglobulin family. MHC class I molecules consist of a heavy chein non-covalently associated with b2-microglobulin. The a1 and a2 domains make up the peptide binding domain, followed by an immunoglobulin domain (a3), a transmembrane region and a cytoplasmic tail. MHC class II molecules are more symmetrically built, with both a and b chains contributing to peptide binding and spannning the membrane. |
 |
|
|
|||||||||||||||||
|
Last Modified by A.Honegger |
|||||||||||||||||