|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Calmodulin
Flexibility of Calmodulin
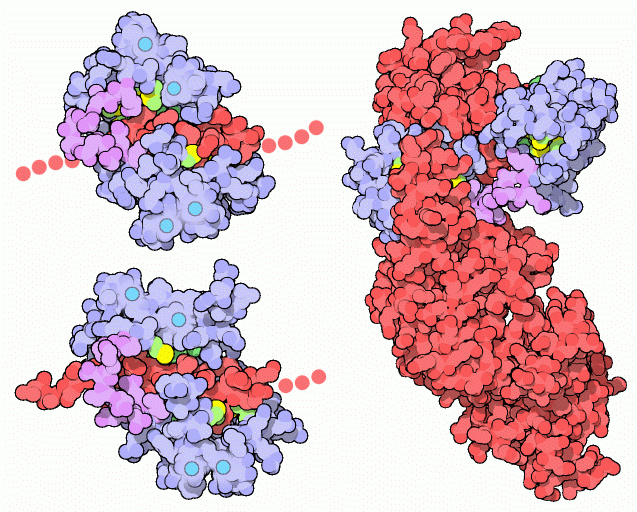
NMR studies clearly show that the connector between the two calcium binding globular domains is flexible even when it is not bound to its target proteins. However, the full range of flexibility can be seen in calmodulin's interactions with its target proteins. Calmodulin typically wraps around its target, with the two globular domains gripping either side of it. The two structures on the left show calmodulin bound to two different target enzymes: calmodulin-dependent protein kinase II-alpha at the top (from PDB entry 1cm1) and myosin light chain kinase on the bottom (from PDB entry 2bbm). In both cases, only a small piece of the target protein chain (shown in red) is included in the crystal structure. Notice how the flexible linker of calmodulin, shown in purple, allows calmodulin to conform to the slightly different shapes of these two targets. A different binding geometry is seen in the edema factor toxin from the anthrax bacteria, shown here on the right from PDB entry 1k93. The toxin is shown in red. Once calmodulin binds to the toxin, a conformational change in the toxin activates its adenylyl cyclase activity, which then depletes the host cell's energy stores. Since calmodulin is absent in bacteria, the anthrax bacteria have cleverly evolved to exploit the abundance of calmodulin in their hosts in order to trigger the toxin and take control of their cellular machinery.Next: Exploring the Structure
Previous: A Functionally Versatile Machine
Last changed by: A.Honegger,