|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Calmodulin
A Functionally Versatile Molecule
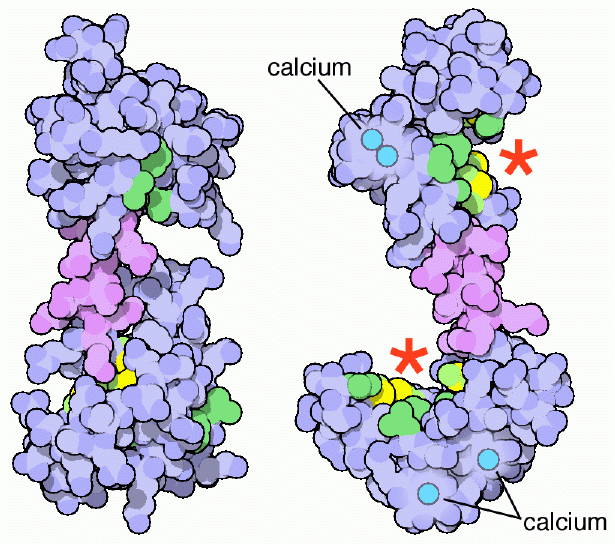
Calmodulin's target proteins come in various shapes, sizes and sequences and are involved in a wide array of functions. For example, calcium-bound calmodulin forms a critical subunit for the regulatory enzyme phosphorylase kinase, which in turn is a regulator for glycogen breakdown. Calmodulin also binds and activates other kinases and phosphatases that play significant roles in cell signaling, ion transport and cell death. One common theme in the contact between calmodulin and its different target proteins is the use of non-polar interactions, in particular, through the interactions with the unusually abundant methionines of calmodulin. Calcium binding exposes these non-polar surfaces of calmodulin, which then bind to non-polar regions on the target proteins. The structure shown on the left, from PDB entry 1cfd, shows calmodulin without calcium, and the structure on the right, from PDB entry 1cll, shows calmodulin after calcium binds. The key nonpolar areas are colored with carbon atoms in green and the many methionine sulfur atoms in yellow. Notice how these non-polar amino acids form two neat grooves (shown with red stars) when calcium binds, waiting to grip the target protein. Because these non-polar grooves are generic in shape, calmodulin acts as a versatile regulatory protein and its targets are not required to possess any specific amino acid sequence or structural binding motifs.Next: Flexibility of Calmodulin
Previous: Structures and Signals
Last changed by: A.Honegger,