|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Calmodulin
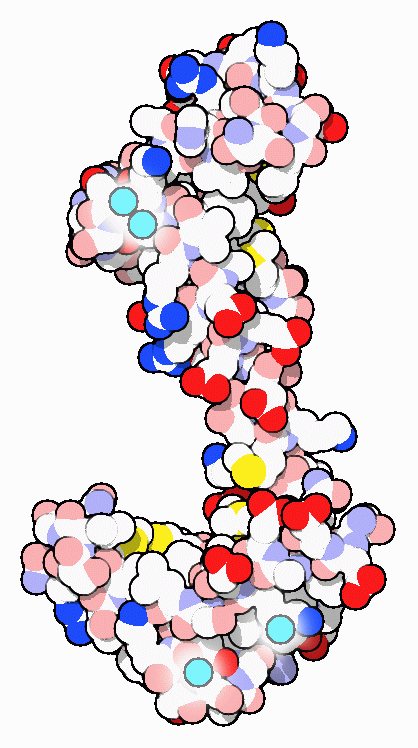
Structures and Signals
Calcium is the most plentiful mineral element found in your body, with phosphorous coming in second. This probably doesn't come as a surprise, since your bones are strengthened and supported by about two kilograms of calcium and phosphorous. Your body also uses a small amount of calcium, in the form of calcium ions, to perform more active duties. Calcium ions play essential roles in cell signaling, helping to control processes such as muscle contraction, nerve signaling, fertilization and cell division. Through the action of calcium pumps and several kinds of calcium binding proteins, cells keep their internal calcium levels 1000-10,000 times lower than the calcium levels in the blood. Thus when calcium is released into cells, it can interact with calcium sensing proteins and trigger different biological effects, causing a muscle to contract, releasing insulin from the pancreas, or blocking the entry of additional sperm cells once an egg has been fertilized.Sensing Calcium
As its name suggests, calmodulin is a CALcium MODULated proteIN. It is abundant in the cytoplasm of all higher cells and has been highly conserved through evolution. Calmodulin acts as an intermediary protein that senses calcium levels and relays signals to various calcium-sensitive enzymes, ion channels and other proteins. Calmodulin is a small dumbbell-shaped protein composed of two globular domains connected together by a flexible linker. Each end binds to two calcium ions. PDB entry 3cln, shown here, has all four sites filled with calcium ions and the linker has formed a long alpha helix separating the two calcium-binding domains.Calmodulin Look-alikes
Many different proteins are sensitive to calcium levels inside (and outside) cells. In the late 1960's, before the discovery of calmodulin, troponin C (see, for instance, PDB entry 1tcf) was the first protein shown to be sensitive to calcium. Troponin C senses rising calcium levels and triggers muscle contraction. The structures of troponin C and calmodulin are remarkably similar, the major difference being the length of the linker connecting the two calcium-binding globular domains. The calcium-binding region of the protein, shown in detail in a later section, is almost identical. This motif has since been found in dozens of other calcium-sensitive proteins.Last changed by: A.Honegger,