 |
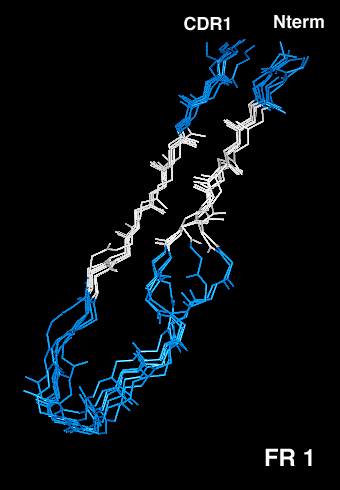
Antibody VH Framework 1
Residues H1-H26
Immunoglobulin VH domain frameworks can be grouped into four distinct types, depending on the main-chain conformation of Framework 1. Based on the analysis of over 200 X-ray structures representing more than 100 non-redundant VH domain sequences, we have come to the conclusion that the marked structural variability of the VH Framework 1 region is caused by three residues: the buried side chain of H6, which can be either a glutamate or a glutamine, the residue in position H7, which may only be a proline if H6 is a glutamine, and H10 (Kabat H9), which has to be either a glycine or a proline if H6 is a glutamate. In natural antibodies, these three residues are encoded in combinations which are compatible with each other and with the rest of the structure and therefore will yield functional molecules. However, the degenerate primer mixtures commonly used for PCR cloning of antibody fragments can and frequently do introduce out-of-context mutations to non-natural combinations which severely reduce stability, production yield and antigen affinity of the scFv.
The aligned VH structures have been color-coded according to the H6/H7/H10 residue combination predictive for the structural subtype:
- Type I: H6=Glu, H7#Pro, H10=Pro, magenta
- Type II: H6=Glu, H7#Pro, H10=Gly, pink
- Type III: H6=Gln, H7#Pro, H10=any amino acid, cyan
- Type I: H6=Gln, H7=Pro, H10=any amino acid, blue
-
-
- Honegger, A. and Plückthun, A., The Influence of the Buried Glutamine or Glutamate Residue in Position 6 on the Structure of Immunoglobulin Variable Domains, J.Mol.Biol, 309 (2001)687-699

-
- Jung, S., Spinelli, S., Schimmele, B., Honegger, A., Pugliese, L., Cambilleau, C. and Plückthun, A., The importance of framework residues H6, H7 and H10 in antibody heavy chains: experimental evidence for a new structural subclassification of antibody VH domain,
- J.Mol.Biol., 309 (2001) 701-716

|