|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Importins
Exploring the Structure
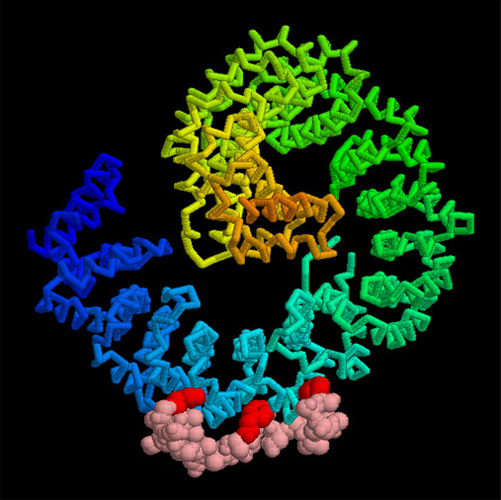
The actual mechanisms that importins use to pull molecules through the nuclear pore are still a subject of active debate, but PDB entry 2bpt gives some hints of how it might be done. The thousands of proteins that make up the nuclear pore are covered with special amino acid sequences that are flexible and that contain many phenylalanines. One side of importin-beta binds to these special sequences. The structure shown here includes the full importin-beta (in rainbow-colored cylinders) and a few short pieces from the nuclear pore proteins (shown in spheres at the bottom, with the phenylalanine amino acids in red). Notice that the phenylalanines bind in pockets on the outer surface of the importin. Importin-beta may jump from site to site through the nuclear pore, guided by these special sequences.
As you take a look at these structures yourself, take a moment to explore the unusual fold of the chain. Importin-beta is folded like a spring, which is then wraps into a big spiral that traps its binding partners inside.
For more information on nuclear pore proteins, a list of 'importin' related entries in the PDB as determined by a keyword search on December 29, 2006 is available. To find the technical articles that I used to write this Molecule of the Month, click here.
Next: Jmol Animation
Previous: Exit Strategy
Last changed by: A.Honegger,