|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Kinesin
Exploring the Structure
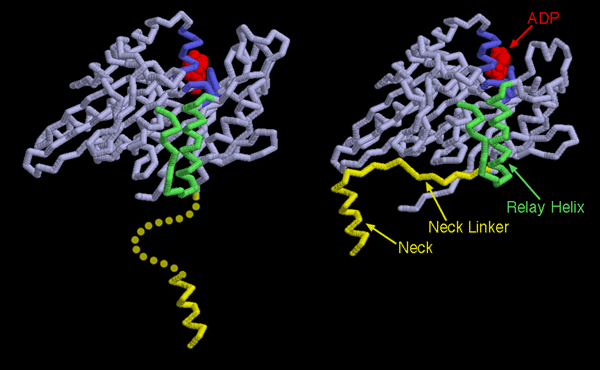
Now that the structures of several molecular motors have been determined, we can see that the mechanism of powered motion is very similar in different kinesins, and remarkably, in myosin as well. The trick used by molecular motors is to link the small change of breaking the ATP phosphate-phosphate bond into a large structural change in the motor. Two kinesin structures are shown here: the one on the left shows kinesin before the power stroke (PDB entry 1bg2), and the one on the right is after the power stroke (PDB entry 2kin). In each case, only one motor domain is shown and ADP is shown in red. The ATP-binding portion, colored blue here, changes slightly as the ATP is cleaved and the phosphate dissociates. The small change pushes on the relay helix, shown here in green, causing it to change slightly in shape. This forms a perfect pocket for the neck linker, shown in yellow. Before the power stroke, the pocket is too small and the linker is flexible and disordered. After the power stroke, the pocket is the right size for the linker to zipper into the protein, dragging along the neck and any cargo attached to it.This illustration was created with RasMol. You can explore these structures and create similar illustrations by clicking on the accession codes and picking one of the options under View Structure.
A list of all Kinesin entries in the PDB as determined by a FASTA search on March 28, 2005 is available here. For more information on kinesin, click here.
Next: Interaktive 3D-Animation
Previous: This Way or That?
Last changed by: A.Honegger,