|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Phenylalanine Hydroxylase
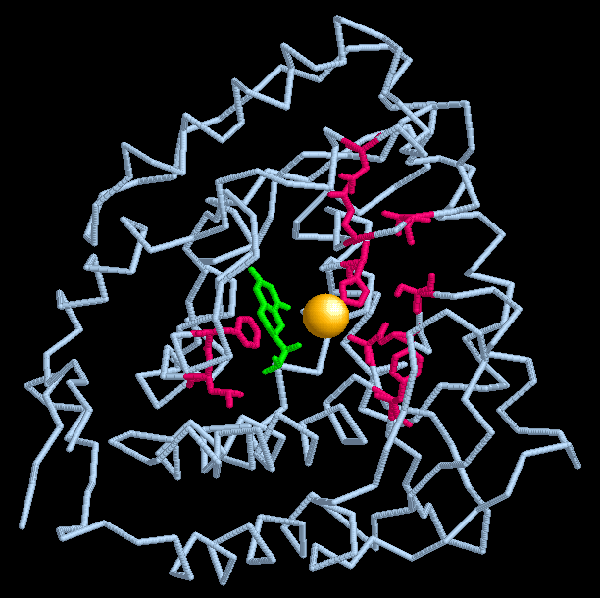
Exploring the Structure
The hydroxylation of phenylalanine requires free oxygen and a helper molecule (cofactor), tetrahydrobiopterin. Although the exact mechanism of the enzyme action is not known, it is clear that the cofactor interacts with a few conserved residues in the enzyme and the primary function of the iron ion is to stabilize this cofactor. The backbone structure of the catalytic domain of phenylalanine hydroxylase is shown here (PDB entry 1j8u) with the tetrahydrobiopterin cofactor (colored green) and the iron ion (yellow sphere). During the course of the reaction the cofactor loses two of its hydrogen atoms to form dihydrobiopterin (as can be seen in PDB entry 1dmw). Yet another enzyme acts on dihydrobiopterin to restore the original form of the cofactor, which is then used in another cycle of hydroxylation.Mutations in phenylalanine hydroxylase can block the conversion of phenylalanine to tyrosine. Several hundred mutations have been documented in this enzyme. Many of these mutations destroy the enzyme activity and lead to phenylketonuria. A few examples are shown here in red. These mutations disrupt the interaction of the enzyme with either the cofactor or the iron ion, reducing or stopping the enzyme activity. Other serious mutations (not shown here) are found in amino acids that stabilize the structure of the enzyme, on the face that forms the enzyme tetramer or in regions that interact with the regulatory domain.
This illustration was created with RasMol. You can create similar pictures of phenylalanine hydroxylase by clicking on the accession codes here, and picking one of the options under View Structure.
A list of all phenylalanine hydroxylase entries in the PDB as determined by a FASTA search on January 1, 2005 is available here. For more information on phenylalanine hydroxylase, click here.
Next: Interaktive 3D-Animation
Previous: Aromatic Alliance
Last changed by: A.Honegger,