|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Catalase
![]()
Living with oxygen is dangerous. We rely on oxygen to power our cells, but oxygen is a reactive molecule that can cause serious problems if not carefully controlled. One of the dangers of oxygen is that it is easily converted into other reactive compounds. Inside our cells, electrons are continually shuttled from site to site by carrier molecules, such as carriers derived from riboflavin and niacin. If oxygen runs into one of these carrier molecules, the electron may be accidentally transferred to it. This converts oxygen into dangerous compounds such as superoxide radicals and hydrogen peroxide, which can attack the delicate sulfur atoms and metal ions in proteins. To make things even worse, free iron ions in the cell occasionally convert hydrogen peroxide into hydroxyl radicals. These deadly molecules attack and mutate DNA. One theory, still controversial, is that this type of oxidative damage accumulates over the years of our life, causing us to age.
Antioxidants to the Rescue
Fortunately, cells make a variety of antioxidant enzymes to fight the dangerous side-effects of life with oxygen. Two important players are superoxide dismutase, which converts superoxide radicals into hydrogen peroxide, and catalase, which converts hydrogen peroxide into water and oxygen gas. The importance of these enzymes is demonstrated by their prevalence, ranging from about 0.1% of the protein in an Escherichia coli cell to upwards of a quarter of the protein in susceptible cell types. These many catalase molecules patrol the cell, counteracting the steady production of hydrogen peroxide and keeping it at a safe level.Better, Stronger, Faster
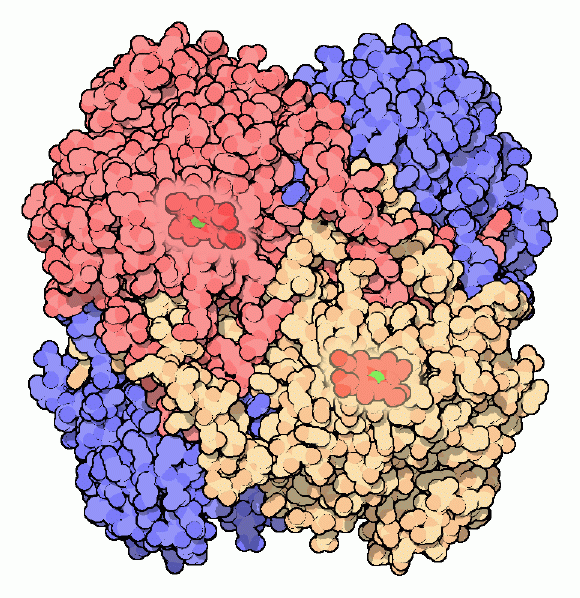
Catalases are some of the most efficient enzymes found in cells. Each catalase molecule can decompose millions of hydrogen peroxide molecules every second. The cow catalase shown here (PDB entry 8cat) and our own catalases use an iron ion to assist in this speedy reaction. The enzyme is composed of four identical subunits, each with its own active site buried deep inside. The iron ion, shown in green, is gripped at the center of a disk-shaped heme group. Catalases, since they must fight against reactive molecules, are also unusually stable enzymes. Notice how the four chains interweave, locking the entire complex into the proper shape.Next: Types of Catalase
Last changed by: A.Honegger,