|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
The Glycolytic Enzymes
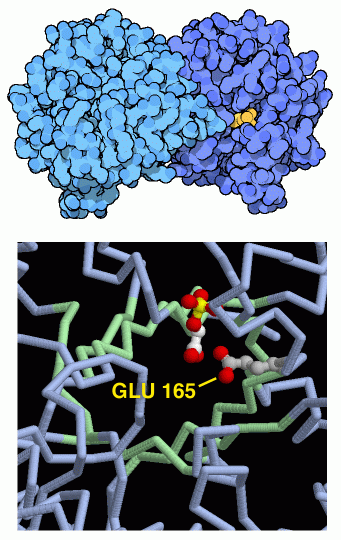
Triose Phosphate Isomerase
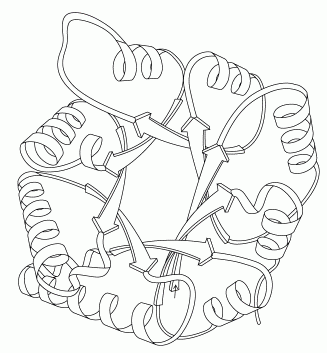
At this stage in glycolysis, the cell has broken the sugar into two different pieces. For economy, it would be ideal to proceed along a single path, instead of requiring a separate path for each of the pieces. The fifth step makes this possible by interconverting the two pieces. Triose phosphate isomerase, shown here from PDB entry 2ypi, pulls a hydrogen atom off one carbon atom and replaces it on a neighboring carbon atom. A special glutamate amino acid, shown in the RasMol picture at lower right, performs the transfer. Triose phosphate isomerase has been described as a perfect enzyme. It performs its reaction billions of times faster than would happen without it. It is so fast that the rate of the reaction is determined by how fast the molecules can get to the enzyme.When you are looking at this structure yourself, also notice that the active site is in the middle of a "beta barrel:" a cylindrical arrangement of extended strands, all colored green in the RasMol picture. The picture shown below, drawn by Jane Richardson, shows the folding of the chain in the protein, with an inner ring of extended beta strands surrounded by an outer ring of alpha helices. Notice how each of the helices connects two neighboring strands in the barrel. As you are exploring the structures of the ten glycolytic enzymes, notice that several other enzymes are also constructed with this beautiful folding pattern.
Last changed by: A.Honegger,