|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Trypsin
The Perils of Proteases
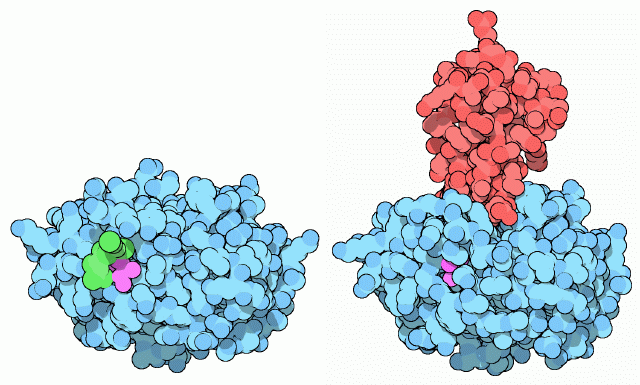
As you might imagine, the digestion of proteins in your body is a delicate business. Protein makes up about one fifth of the material in each of your cells, so you must be careful when creating protein-cutting machines. For digestive enzymes, the trick is to create the enzyme in an inactive form (termed a zymogen), and then to activate it once it is in the intestine. Trypsin is built with an extra piece of protein chain, colored in green in the structure on the left (PDB entry 1tgs). Actually, only two amino acids of this extra bit are seen in crystal structure, so you have to imagine the rest flopping around away from the protein. This longer form of trypsin, called trypsinogen, is inactive and cannot cut protein chains. Then, when it enters the intestine, the enzyme enteropeptidase makes one cut in the trypsin chain, clipping off the little tail. This allows the new end of the chain, colored here in purple, to tuck into the folded protein and stabilize the active form of the enzyme, as shown on the right (PDB entry 2ptc). As extra insurance, the pancreas also makes a small protein, trypsin inhibitor (shown in red), that binds to any traces of active trypsin that might be present before it is secreted into the intestine. It binds to the active site of trypsin, blocking its action but not itself being cut into tiny pieces.Next: Exploring the Structure
Previous: Trypsin
Last changed by: A.Honegger,