|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Trypsin
Your body needs a steady supply of amino acids for use in growth and repairs. Each day, a typical adult needs something in the range of 35-90 grams of protein, depending on their weight. Quite surprisingly, a large fraction of this may come from inside. A typical North American diet may contain 70-100 grams of protein each day. But your body also secretes 20-30 grams of digestive proteins, which are themselves digested when their finish their duties. Dead intestinal cells and proteins leaking out of blood vessels are also digested and reabsorbed as amino acids, showing that our bodies are experts at recycling.
Protein Scissors
Proteins are tough, so we use an arsenal of enzymes to digest them into their component amino acids. Digestion of proteins begins in the stomach, where hydrochloric acid unfolds proteins and the enzyme pepsin begins a rough disassembly. The real work then starts in the intestines. The pancreas adds a collection of protein-cutting enzymes, with trypsin playing the central role, that chop the protein chains into pieces just a few amino acids long. Then, enzymes on the surfaces of intestinal cells and inside the cells chop them into amino acids, ready for use throughout the body.The Protein-Cutting Machinery
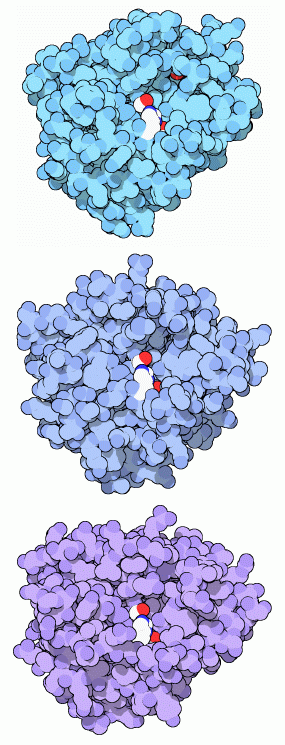
Trypsin uses a special serine amino acid in its protein-cutting reaction, and is consequently known as a serine protease. The serine proteases are a diverse family of enzymes, all of which use similar enzymatic machinery. In digestion, trypsin, chymotrypsin and elastase work together to chop up proteins. Each has a particular taste for protein chains: trypsin (shown at the top from PDB entry 2ptn) cuts next to lysine and arginine, chymotrypsin (shown in the middle from PDB entry 2cha) cuts next to phenylalanine and other large amino acids, and elastase likes chains with small amino acids like alanine (shown at the bottom from PDB entry 3est). In each picture, the key serine is shown at center in red, with a histidine (white and blue) and an aspartate (only one red oxygen can be seen) highlighted below it. Trypsin-like enzymes are also found in many other places in the body. Some of these are highly specific, cleaving only a specific target protein. For instance, thrombin, presented in the Molecule of the Month in January 2003, is designed to make a specific cut in fibrinogen, creating a blood clot.Sturdy Enzymes
Serine proteases played a central role in the discovery and study of enzymes. This is because they are particularly easy to study. They are plentiful in digestive juices and very stable, so they are relatively easy to collect and purify. It is also easy to study their function: you just toss in some protein and see how fast it is digested. Chymotrypsin was among the first proteins to be studied by X-ray crystallography, revealing its complex machinery for holding the protein targets and performing a precise atomic change. Today, there are hundreds of structures of serine proteases available in the PDB, waiting to be explored.Next: The Perils of Proteases
Last changed by: A.Honegger,