|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Src Tyrosine Kinase
Many Moving Parts
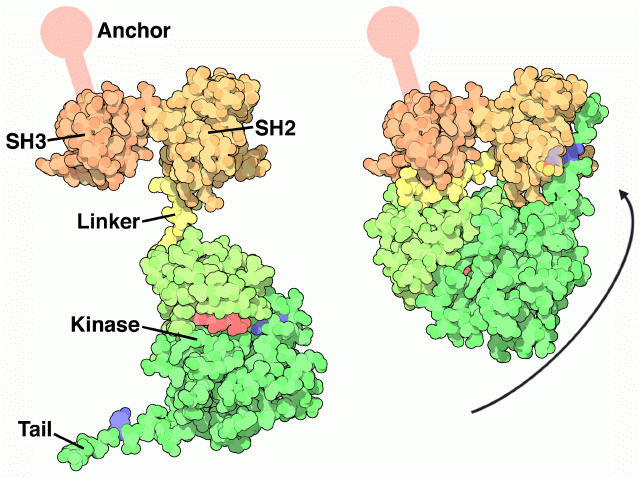
Src undergoes a large articulated motion when it turns "on" and "off." The crystal structure, shown on the right from PDB entry 2src, shows the inactive form. The protein opens up, as shown on the left, to form the active protein. The Src protein is composed of a string of functional parts connected together in a single protein chain. Walking from one end of the chain to the other, it is composed of an anchoring segment (not seen in the crystal structure), an SH3 domain, an SH2 domain, a flexible linker, a kinase domain, and a final tail.Each of these parts is essential for the function. Look first at the active form on the left. The SH3 domain has a little groove that grabs protein chains, positioning them close enough to the kinase domain to allow the addition of phosphate groups. The ATP shown here in red provides the phosphate groups. The inactive form, shown on the right, is shut down in a clever way. It folds tightly upon itself, using the short linker to block the SH3 domain so that it cannot bind to other proteins. The key to the transition is a tyrosine in the tail, colored blue here. When this tyrosine is phosphorylated, it binds in the SH2 domain, gluing the whole complex shut. When the phosphate is removed, the tail is released and the whole molecule opens up, unblocking the SH3 binding site and allowing access to the kinase active site.
Next: Exploring the Structure
Previous: Src Tyrosine Kinase
Last changed by: A.Honegger,