|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Chaperones
Exploring the Structure
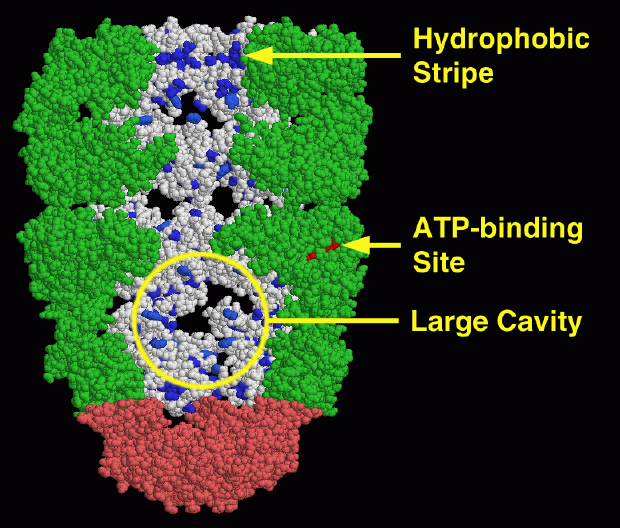
The large GroEL-GroES complex is available in PDB entry 1aon. In this picture, three of the subunits in each GroEL ring have been removed to show the interior, leaving four subunits in each ring. On the two in back, the carbon-rich amino acids, LEU, ILE, VAL, MET, PHE, TYR and TRP, are colored blue. Notice the stripe of carbon-rich "hydrophobic" amino acids around the entry at the top. This will interact strongly with unfolded proteins by coaxing them into the cavity. Now look at the bottom cavity, capped by the pink GroES at the bottom. Powered by ATP (ADP is found in this structure, colored bright red here), the ring of GroEL undergoes a major change in shape. The cavity is much larger, and the stripe of carbon-rich amino acids is hidden from the cavity. This forces a protein chain trapped inside (not shown in the picture) to fold on its own, giving it plenty of room for the process.This picture was created with RasMol. You can create similar pictures by clicking on the accession codes given here and picking one of the options under View Structure.
For more information on chaperones, click here.
Next: Interaktive 3D-Animation
Previous: HSP-70 and Prefoldin
Last changed by: A.Honegger,