|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Chaperones
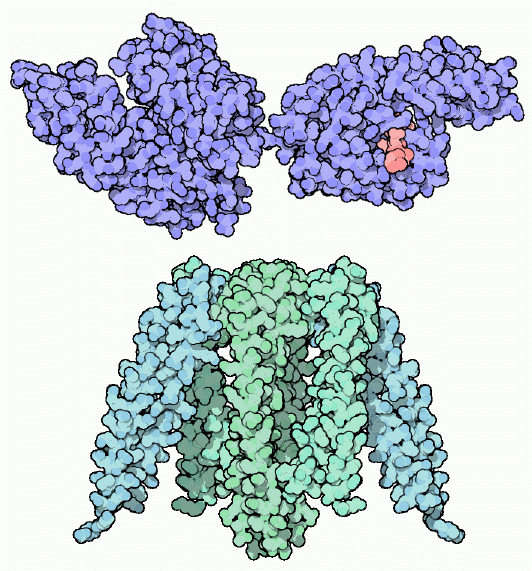
HSP-70 and Prefoldin
Smaller chaperones protect proteins just after they leave the ribosome. At this stage, they may have very little folded structure, so stretches of the chain with lots of exposed carbon atoms are particularly susceptible to aggregation. HSP-70 (shown at the top) finds these stretches and binds to them, shielding them from neighbors. Then, powered by ATP, the chaperone releases the chain when it is ready to fold. HSP-70 is composed of two domains: one that binds ATP and controls the process, shown on the left side of the molecule from PDB entry 1dkg, and one that binds to carbon-rich peptides, shown here on the right side using coordinates from PDB entry 1dkz. A little peptide, colored pink, is bound in the deep protein-binding cleft. The odd jelly-fish shaped prefoldin, shown at the bottom from PDB entry 1fxk, performs a similar job, engulfing protein chains when they are in the process of folding.Next: Exploring the Structure
Previous: Chaperones
Last changed by: A.Honegger,