|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Myosin
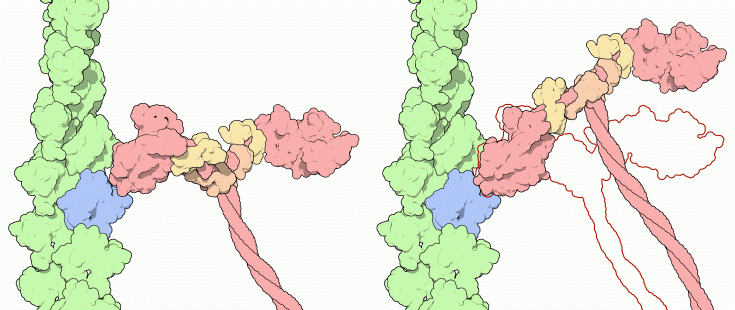
The Power Stroke
ATP contains a key phosphate-phosphate bond that is difficult to create and is used to power many processes inside cells. You might be surprised to find, however, that breakage of this phosphate-phosphate bond is not directly responsible for the power stroke in myosin. Instead, it is release of the phosphate left over after ATP is cleaved that powers the stroke. Think of myosin like an arm that can flex towards you or push away. The cleavage of ATP is used in a priming step. When ATP is cleaved, myosin adopts a bent, flexed form, like in the structure on the left (PDB entry 1br1). This prepares myosin for the power stroke. The flexed myosin then grabs the actin filament (shown in green and blue, from PDB entry 1atn) and release of phosphate snaps it into the straight "rigor" form, as shown on the right (PDB entry 2mys). This power stroke pushes the myosin molecule along the actin filament. When finished, the remaining ADP is replaced by a new ATP, the myosin lets go of the actin filament. Then, it is ready for the next stroke.Note that these illustrations only show the proteins--the ATP, ADP and phosphate molecules are bound inside the proteins and are not visible from this view. To see the location of the nucleotides, go on to the next page.
Next: Exploring the Structure
Previous: Power in Numbers
Last changed by: A.Honegger,