|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Selenocysteine Synthase
Exploring the Structure
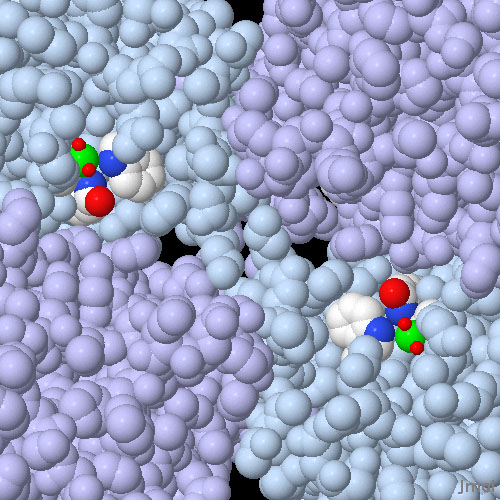
About 15 selenoproteins have been found in human cells. These include several deiodinase enzymes that are essential for the generation of thyroid hormones, several glutathione peroxidases that are important for the conversion and detoxification of compounds with reactive oxygen atoms, and several proteins of unknown function. The active site of glutathione peroxidase is shown here from PDB entry 1gp1. The picture shows two of the four identical active sites in the tetrameric enzyme. Each active site is composed of a selenocysteine amino acid (the selenium atoms are colored green) which is activated by a tryptophan and a glutamine (shown with atomic colors). In this crystal structure, the selenocysteine is highly oxidized, with two oxygen atoms (shown as small red spheres) bound to the selenium. In the normal catalytic cycle of the enzyme, it is thought that the selenium extracts one reactive oxygen from dangerous peroxides, and then uses water and glutathione to neutralize it.
This illustration was created with Jmol--you can see an interactive version of the structure by clicking on the image. To see the scientific articles used for this Molecule of the Month, click here. Also available are related entries in the PDB as determined by a keyword search on July 30, 2008 for "Selenocysteine Synthase" OR SelB.
Topics for further exploration
- There are several natural selenoproteins in the PDB, as well as many proteins with selenomethionine residues that were used in the crystallographic structure determination. Can you find some examples?
- SelB recognizes the special insertion sequence by forming specific contacts with a guanine base in the mRNA loop. Can you identify the amino acids that are important for this recognition?
Previous: Specifying Selenium
Next: Jmol Animation
Last changed by: A.Honegger,