|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Citrate Synthase
Your body burns up a lot of food every day. However, cells don't burn food like a fireplace. Instead, food molecules are combined with oxygen molecules one-by-one, in many carefully controlled steps. In this way, the energy that is released can be captured in convenient forms, like ATP or NADH, which are then used elsewhere to power essential cellular functions. Our cells get most of their energy from a long series of reactions that combine oxygen and glucose, forming carbon dioxide and water, and creating lots of ATP and NADH in the process.
Making the Link
Citrate synthase is a central enzyme in this process of sugar oxidation. It is the first step of the citric acid cycle, also known as the Krebs cycle. Glucose has previously been broken into several pieces by glycolysis, releasing two carbon atoms as carbon dioxide and leaving the rest as two molecules of acetate, carried in an activated form on special cofactor molecules. In the citric acid cycle, these remaining carbon atoms are fully oxidized to form carbon dioxide. Citrate synthase starts this process by taking the molecules of acetate and attaching them to oxaloacetate, which acts as a convenient handle as the carbon atoms are passed from enzyme to enzyme in the citric acid cycle.
A Perfect Fit
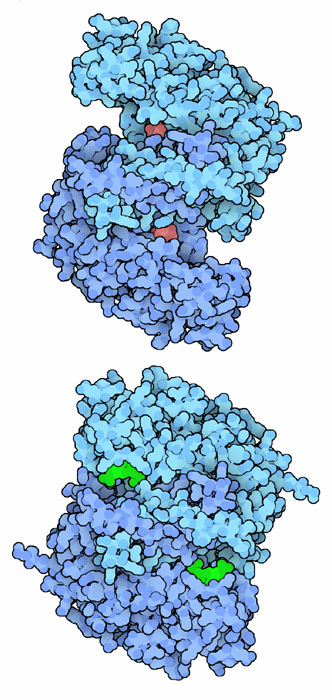
Citrate synthase is a classic example of induced fit in enzyme action. The enzyme from pigs is shown here, from PDB entry 1cts and 2cts . The enzyme in our cells and in most animals and plants is very similar. It is composed of two identical subunits, each with its own active site. The enzyme starts in an open form (shown at the top, with the product citrate in red), and when it binds to its substrates, it closes around them and performs the linkage reaction (shown at the bottom, with the two substrates in green).
Last changed by: A.Honegger,