|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Thymine Dimers
Summer is here, and we're all heading outdoors to enjoy the sun. But remember to take your sunscreen, since too much sunlight can damage your cells. Small doses of sunlight are needed to create vitamin D, but larger doses attack your DNA. Ultraviolet light is the major culprit. The most energetic and dangerous wavelengths of UV light, termed UVC, are screened out (at least for now) by the ozone in the upper atmosphere. However, the weaker UV light, termed UVA and UVB, passes through the atmosphere and is powerful enough to cause chemical changes in the DNA.
Dangerous Dimers
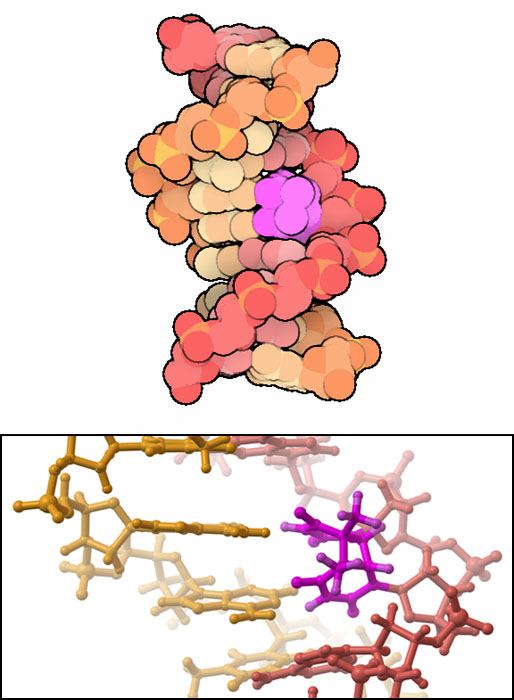
Ultraviolet light is absorbed by a double bond in thymine and cytosine bases in DNA. This added energy opens up the bond and allows it to react with a neighboring base. If the neighbor is another thymine or cytosine base, it can form a covalent bond between the two bases. The most common reaction is shown here: two thymine bases have formed a tight thymine dimer, with two bonds gluing the bases together. The upper image is from PDB entry 1n4e and the close-up picture at the bottom is from PDB entry 1ttd. This is not a rare event: every second you are in the sun, 50 to 100 of these dimers are formed in each skin cell!
Problems with Polymerases
These dimers are awkward and form a stiff kink in the DNA. This causes problems when the cell needs to replicate its DNA. DNA polymerase has trouble reading the dimer, since it doesn't fit smoothly in the active site. TT dimers like the ones shown here are not the major problem, since they are usually paired correctly with adenine when the DNA is replicated. But CC dimers do not fare as well. DNA polymerase often incorrectly pairs adenine with them instead of guanine, causing a mutation. If this happens to be in an important gene that controls the growth of cells, such as the genes for Src tyrosine kinase or p53 tumor suppressor, the mutation can lead to cancer.
Last changed by: A.Honegger,