|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Aconitase and Iron Regulatory Protein 1
Exploring the Structure
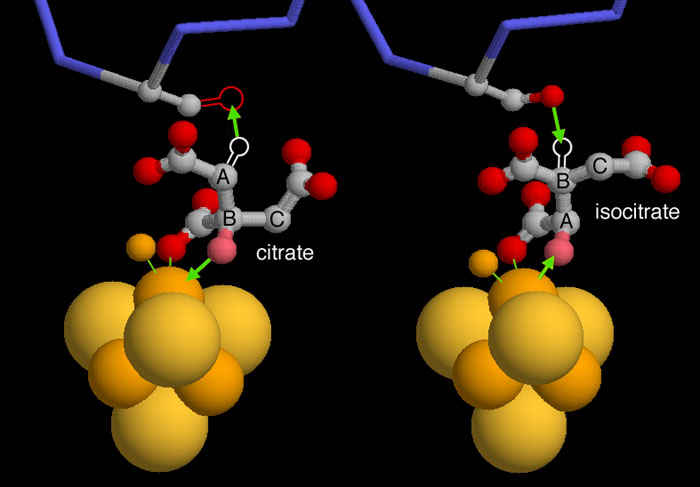
Aconitase performs a classic stereospecific reaction that is often used as an example in biochemistry textbooks. It extracts a hydroxyl group and a specific hydrogen atom from citrate, and replaces them in a geometrically precise way to form isocitrate. This process is revealed in two crystal structures, but you need to use a little imagination when you look at them, since the crystal structures do not contain the hydrogen atom positions.
PDB entry 1c96, shown on the left, has citrate bound in the active site. In the normal form of the enzyme, the oxygen atom shown in pink will be extracted by the iron sulfur cluster and a hydrogen atom will be extracted by a serine at the top (both of these reactions are shown with green arrows). This structure, however, has mutated the serine to alanine, so the oxygen atom in the serine is missing. In the second step of the reaction, shown on the right from PDB entry 7acn, the molecule flips upside down (notice the different location of the labels A-B-C) and the hydrogen and hydroxyl are added back in different places to form isocitrate.
These illustrations were created with RasMol. You can create similar illustrations by clicking on the accession codes here and picking one of the options under Images and Visualization.
To see some of the journal articles used to write this Molecule of the Month, click here.
Also available are related entries in the PDB as determined by keyword searches on April 27, 2007 for 'aconitase' and for 'iron regulatory protein 1'.
Next: Jmol Animation
Previous: Moonlighting Proteins
Last changed by: A.Honegger,