|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Clathrin
With its intricate meshwork of protein braids and alluring symmetry, clathrin is sure to seize your attention. It was named in the 1960s for its clathrate (lattice of bars) appearance in electron micrographs, and to this day, this beautiful molecule invokes intensive study. Like many proteins, clathrin represents a perfect case of form following function; it performs critical roles in shaping rounded vesicles for intracellular trafficking.
Intracellular Traffic
Most of the cells in your body are divided into a variety of membrane-sealed compartments called organelles. Each compartment has a membranous skin, constructed of lipids and proteins, that encapsulates a specialized environment. The molecules inside each compartment perform specific tasks and must remain isolated from the cytoplasm, both to enhance their chemical efficiency and in many cases to protect the cytoplasm from potentially harmful functions. For instance, degradative enzymes destroy their target molecules inside lysosomes, oxidative enzymes are sealed in peroxisomes, and cell-suicide activators are sequestered inside the intermembrane spaces of mitochondria. Although segregated, these compartments must communicate with one another, for example, to refresh aging proteins and to shuttle the reactants and products of cell metabolism. Cells can safely transport molecules over great distances in small vesicles that bud into the cytoplasm from an organelle's surface, and then fuse with the target organelle. In most cases these vesicles do not form spontaneously, but rather, are facilitated by coat-proteins like clathrin. Cells endocytose vesicles of various sizes from the plasma membrane, for example, to take in nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. In some cases, however, the endocytic mechanism also provides a pathway for raiding pathogens or toxins!
Rapid Transit
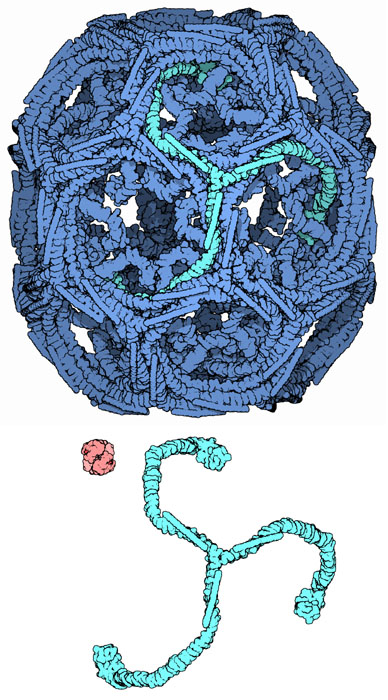
It takes less than a minute to form a typical clathrin-coated vesicle. This may sound slow compared to the rates of many enzyme reactions, but, since they need to transport bags of cargo (lipids and proteins) in bulk quantities, the macromolecular cages formed by clathrin are enormous when measured against typical enzymes; you can contrast the size of clathrin to a hemoglobin molecule, shown here in red. A macrophage, for example, internalizes an equivalent of 100% of its membrane roughly every 30-60 minutes as it samples its environment via small vesicles from its cell membrane, while a hepatocyte uses vesicles to secrete a quantity of serum proteins like transferrin and LDL equivalent to its total protein content every day. After a vesicle buds into the cytoplasm, the coat rapidly disassembles, allowing the clathrin to recycle while the vesicle gets transported to a variety of fates.
Molecular Basket-weaver
Clathrin cages are composed of symmetrical three-legged components called triskelions. The structure shown here, PDB entry 1xi4, is built of 36 triskelia, one of which is highlighted in green. When triskelia snap together in solution, they can interact with enough flexibility to form either 6-sided rings that yield a flatter surface, or 5-sided rings with higher curvature. In a cell, a triskelion floating in the cytoplasm binds to an adaptor protein (shown on the next page), linking one of its three feet to the membrane at a time. This triskelion will bind to other membrane-attached triskelia to form a rounded lattice of hexagons and pentagons, reminiscent of the panels on a soccer ball, that pulls the membrane into a bud. By constructing different combinations of 5-sided and 6-sided rings, vesicles of different sizes may assemble. The structure shown here represents the second smallest possible cage structure, which is actually too small to contain a functional vesicle. It was created in the laboratory by reconstituting triskelions without a lipid vesicle. The smallest clathrin cage commonly photographed, called a mini-coat, has 12 pentagons and only two hexagons. Even smaller cages with zero hexagons probably don't form from the native protein, because the feet of the triskelia are too bulky.
Next: Special Delivery
Last changed by: A.Honegger,