|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Glucose Oxidase
Diabetes is a worldwide health problem affecting hundreds of millions of people. Fortunately, with careful management of diet and medication, the many complications of diabetes can be reduced. Part of this treatment includes the monitoring of glucose levels in the blood, so that proper action may be taken if levels get too high. The enzyme glucose oxidase has made glucose measurement fast, easy, and inexpensive.
Chemical Defense
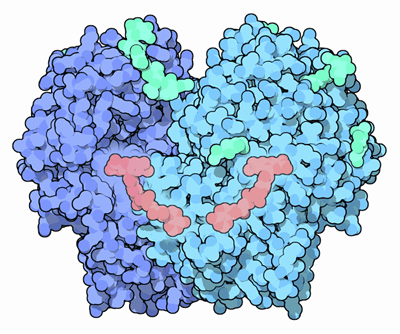
Glucose oxidase, shown here from PDB entry 1gpe, is a small, stable enzyme that oxidizes glucose into glucolactone, converting oxygen into hydrogen peroxide in the process. Its normal biological function appears to be centered on the peroxide that is formed: hydrogen peroxide is a toxic compound that can be used to kill bacteria. For instance, glucose oxidase is found on the surface of fungi, where it helps protect against bacterial infection, and it is also found in honey, where it acts as a natural preservative.
Biotech Bonanza
This obscure enzyme, while playing only a minor role in natural settings, has become the center of a $5 billion biotech industry. It is used as the heart of biosensors that measure the amount of glucose in blood. The trick to these biosensors is that the enzyme takes something that is difficult to measure--glucose--and creates something that is easy to measure--hydrogen peroxide. A typical laboratory glucose meter includes some of the enzyme trapped inside a membrane. Glucose enters the sensor and is converted to glucolactone. In the process, hydrogen peroxide is formed, which is then sensed by a platinum electrode. The more glucose there is in the sample, the more peroxide is formed, and the stronger the signal at the electrode.
Controlling Costs
Of course, platinum electrodes are expensive, so newer biosensors use a serendipitous property of glucose oxidase to make even more affordable glucose meters. Glucose oxidase is very specific for glucose in the initial oxidation reaction, but can use many different compounds as the final electron acceptor, not just oxygen. So, biotech researchers have developed different "mediator" molecules, such as ferrocene, to work in place of oxygen. These molecules pick up electrons from the glucose reaction, and then deliver them directly to a cheaper type of electrode. This method is used to create single-use, disposable test strips that have glucose oxidase, the mediator, and the electrode compound all printed on the surface.
Next: Choices for Biosensors
Last changed by: A.Honegger,