|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Designer Proteins
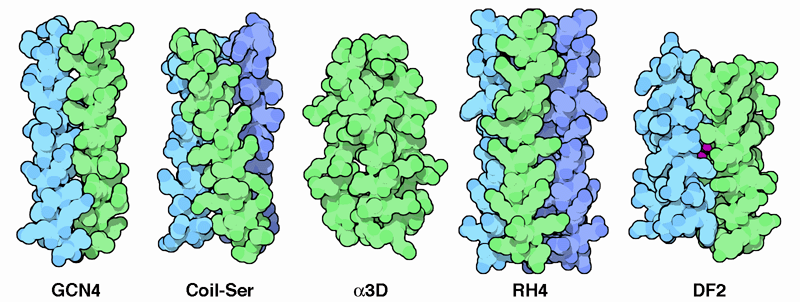
Amazing Alpha Helices
A popular approach to protein design is to start with a natural protein and make changes from there. Many groups have focused on a particularly stable structure composed of alpha helices. The GCN4 protein, a transcriptional activator from yeast, contains two chains that are linked together with a leucine zipper, a special sequence that forms an alpha helix with lots of leucines on one side. The leucines are spaced perfectly so they can zip together, gluing the two alpha helices together, as shown on the left from PDB entry 2zta. Taking this as the start, many groups have designed new proteins based on this principle. Coil-Ser (PDB entry 1cos) is composed of three short protein chains that form alpha helices and then associate into a tight bundle. Alpha3D (PDB entry 2a3d) is composed of a single chain that folds into a bundle of three alpha helices connected by short loops. RH4 (PDB entry 1rh4) was designed, by careful choice of the amino acids on the inside, to remove the characteristic twist of alpha helical bundles--notice that the alpha helices are tipped differently than in the other structures. Finally, researchers are now designing new functions in these artificial proteins, such as adding binding sites for metals in the protein DF2 (PDB entry 1jmb).Next: Exploring the Structure
Previous: Designer Proteins
Last changed by: A.Honegger,