|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Ubiquitin
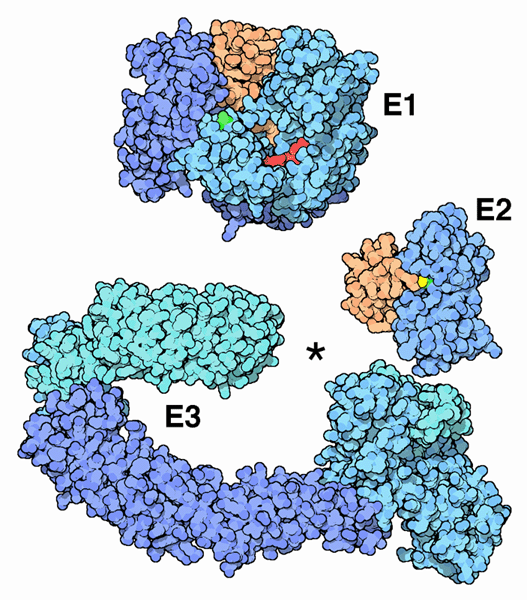
Ubiquitination
The tricky part of this whole process is making sure that ubiquitin is attached only to the proper proteins. Several specialized enzymes sort through the proteins in the cell and pick only the right ones. There are three types of these enzymes, called E1, E2, and E3. The E1 enzyme, shown at the top here from PDB entry 1r4n, is the ubiquitin-activating enzyme that starts the process. Powered by ATP (shown in red), it attaches the tail end of ubiquitin (shown here in light orange) to one of its own cysteine amino acids (shown in green--note, in this structure, the cysteine is mutated to alanine). Then, E1 passes the activated ubiquitin to one of several E2 enzymes, the ubiquitin-conjugating enzymes, shown here from PDB entry 1fxt. These E2 enzymes then work with a large number of different E3 enzymes to recognize obsolete proteins and attach the ubiquitin to them. The E3 enzyme shown here, from PDB entries 1ldk and 1fqv, is shaped like a big clamp. The target protein binds in the gap (shown with the star). The left side of the enzyme recognizes the protein and the right side positions E2 to allow transfer of its ubiquitin.Next: Total Destruction
Previous: Ubiquitin
Last changed by: A.Honegger,