|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Caspases
Billions of cells in your body will die in the next hour. This is entirely normal--the human body continually renews itself, removing obsolete or damaged cells and replacing them with healthy new ones. However, your body must do this carefully. If cells are damaged, like when you cut yourself, they may swell and burst, contaminating the surrounding area. The body responds harshly to this type of cell death, inflaming the area by rushing in blood cells to clean up the mess. To avoid this messy problem, your cells are boobytrapped with a method to die cleanly and quickly on demand. When given the signal, the cell will disassemble its own internal structure and fragment itself into small, tidy pieces that are readily consumed by neighboring cells. This process of controlled, antiseptic death is called apoptosis.
What Cells Must Die?
Apoptosis is used for many purposes. During the development of embryos, organs are shaped by building oversized structures and then pruning back the cells that aren't needed. For instance, during development of the nervous system, half of the neurons die, leaving the proper neural wiring. If you have watched a tadpole lose its tail, you have also seen apoptosis in action. When you are an adult, apoptosis continues its work as obsolete cells die and are replaced by new cells, particularly in organs with high turnover such as the bone marrow and intestines. Apoptosis protects us from cells damaged by radiation or infected by viruses--when detected, these rogue cells are told promptly to commit suicide. Apoptosis is also one of our major defenses against cancer, and deadly cancer cells often have mutations that disable their own apoptosis machinery.The Executioner
Caspases are the executioners of apoptosis. They are protein-cutting enzymes that chop up strategic proteins in the cell. The name refers to two properties of these enzymes. First, they are cysteine proteases that use the sulfur atom in cysteine to perform the cleavage reaction. Second, they cut proteins next to aspartate amino acids in their chains. They do not cut indiscriminantly--instead, they are designed to make exactly the right cuts needed to disassemble the cell in an orderly manner.Caspases in the PDB
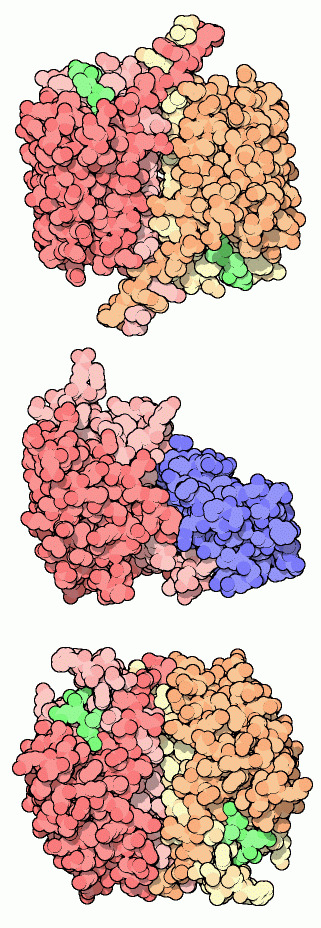
Almost a dozen caspases have been discovered in human cells, each with a slightly different task. Structures of many of them are available in the PDB. Three are shown here. Caspase-1 (also known as interleukin-1beta-converting enzyme) was the first one discovered. It is not involved directly in apoptosis, but instead processes a cell signaling molecule in white blood cells. As with the other caspases, the active form is composed of two chains, each of which is cut into two pieces. The structure shown here at the top, from PDB entry 1ice, has small inhibitors bound in the two active sites, shown in green. Caspase-9 is shown at the center (PDB entry 1nw9), bound to an inhibitory protein (shown in blue). The inhibitor holds it in an inactive form. When it is released, this caspase can link up with another and form the larger active complex. Caspase-9 is an initiator caspase--one that begins the process of apoptosis. It receives the message to begin work, becomes activated, and then makes a cut in the effector caspases, such as caspase-3 shown at the bottom (PDB entry 1pau). Caspase-3, along with other effector caspases, then begin the heavy work of disassembling the cell.For more information on the genomic aspects of caspases, take a look at the Protein of the Month at the European Bioinformatics Institute.
Next: Helpers in Apoptosis
Last changed by: A.Honegger,