|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
The Glycolytic Enzymes
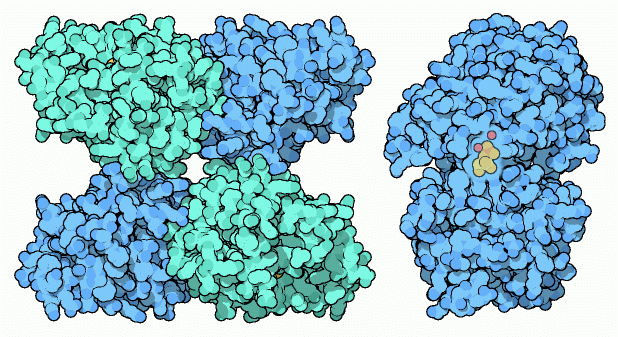
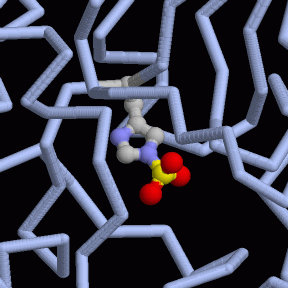
Phosphoglycerate Mutase
The last three steps of glycolysis will remove the remaining phosphates from the two pieces, and use them to make two more molecules of ATP. Phosphoglycerate mutase begins this final capture of energy by shifting the phosphate from the end of the molecule to a strategic place in the center. This enzyme comes in several forms: the yeast enzyme, shown at the left from PDB entry 3pgk, is composed of four identical subunits. Our enzyme is similar, but only contains two subunits. Plants and many bacteria build an entirely different type that uses manganese ions for the reaction, as shown on the right from PDB entry 1eqj.The enzyme in our cells uses a special histidine amino acid, like the one shown to the right from a bacterial enzyme in PDB entry 1e58. This histidine extracts the phosphate and places it back in a different place. Actually, the enzyme does the reverse of this: it first places a phosphate on the molecule, so that two are attached, then it takes off the other one. In order to do this, the enzyme must be charged with this extra phosphate molecule before it can perform its reaction. A small intermediate molecule--2,3- bisphosphoglycerate--delivers these reactive phosphates to the enzyme. After the enzyme is charged, it remains active for a minute or two (busily performing its reaction many times) before the phosphate falls off and has to be replaced.
Next: 10: Enolase
Previous: 8: Phosphoglycerate Kinase
Last changed by: A.Honegger,