|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
The Glycolytic Enzymes

Hexokinase
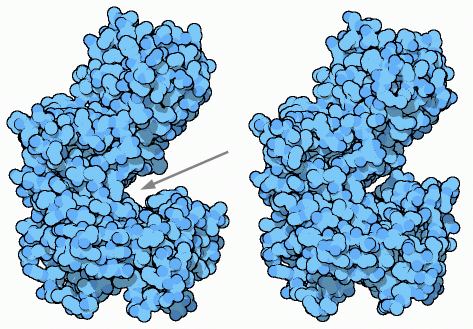
Hexokinase performs the first step in glycolysis, using an ATP molecule to start the process. It transfers a phosphate from ATP to glucose, forming glucose-6-phosphate. Daniel Koshland realized, a decade before the structure was known, that this chemical reaction must be shielded from water, to keep the phosphate from being simply cleaved off of ATP by a water molecule. So he proposed that hexokinase performs an induced fit, closing around ATP and glucose once they are bound. When several structures of yeast hexokinase were solved in the 1970's, this was shown to be true. Hexokinase is shaped like a clamp, with a big groove in one side (shown below with an arrow). The structure without glucose, shown at bottom left from PDB entry 2yhx, is open, allowing access to the active site. But when glucose binds, as shown at bottom center from PDB entry 1hkg, it closes down, surrounding the molecule. These two structures were solved before the amino acid sequence was known, so the structures are incomplete. For an updated view, take a look at entry 1ig8 for the open form, and entry 1bdg (which is a similar form from a blood fluke) for the glucose-bound form.As is often the case, when you look at the human form, things get more complicated. We build several types of hexokinase, made for the slightly different needs of different types of cells. The one shown here on the right is from brain cells, from PDB entry 1dgk. It is twice as big as yeast hexokinase, and is amazingly constructed like two yeast enzymes strung together head-to-tail. Both halves have a nearly identical active site. But, the lower one has specialized in the catalytic reaction, while the upper one has specialized in regulation and does not perform the phosphate-transfer reaction.
Next: 2: Phosphoglucose Isomerase
Previous: The Glycolytic Enzymes
Last changed by: A.Honegger,