|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
The Glycolytic Enzymes
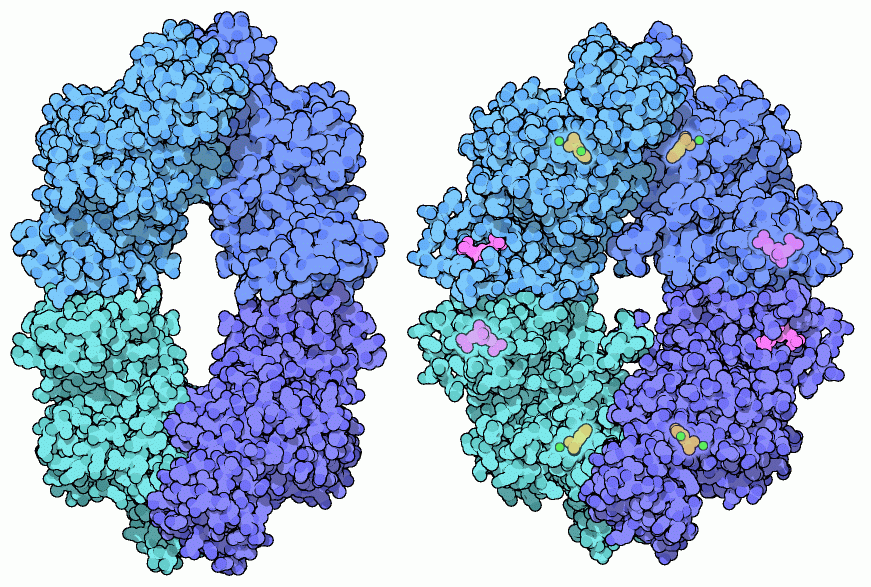
Pyruvate Kinase
In the last step of glycolysis, the cell is finally ready to make a net gain in ATP production. Pyruvate kinase removes the remaining phosphates and places them on ADP, to create new ATP molecules. This allows the unstable little sugar fragments to rearrange into stable pyruvate molecules. These pyruvates leave glycolysis and are subsequently burned up completely into carbon dioxide and water, or converted into throw-away molecules like alcohol or lactic acid.The exit gate of glycolysis is guarded by a pyruvate kinase, ensuring that ATP is made only when needed. Like hemoglobin, it is progressively activated as the levels of its starting materials rise. It is also activated by the presence of phosphorylated sugars, which indicate that plenty of raw materials are available. Conversely, it is inhibited by molecules that are plentiful when the cell has enough energy, such as ATP and amino acids. Pyruvate kinase is an allosteric enzyme that senses the levels of all of these molecules and changes shape based on the result. It is composed of four flexible subunits arranged in a diamond shape. The whole complex flexes as different molecules bind. The active site is shown near the top and bottom here, and a separate regulatory site is more towards the center. The bacterial structure shown on the left, from PDB entry 1e0u, is in the inactive state. The yeast structure on the right, from PDB entry 1a3w, has a molecule bound in the regulatory site, shown in magenta, and has flexed into the active shape. The active site contains two metal ions, a potassium ion and a magnesium ion shown here in green, that assist with the reaction.
Next: Interaktive 3D-Animation
Previous: 10: Enolase
Last changed by: A.Honegger,