|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Carbonic Anhydrase
Exploring the Structure
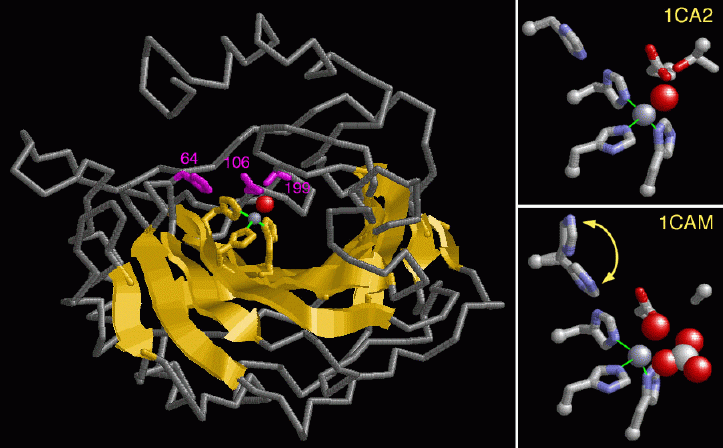
The alpha carbonic anhydrase enzymes have been well studied, leading to an understanding of how the enzyme works. The left hand figure shows the structure of carbonic anhydrase II from PDB entry 1ca2. Note the large beta sheet in the center of the structure colored in yellow. The active site lies at the bottom of a deep cleft in the enzyme where a zinc atom is bound, shown with a gray sphere. Nitrogen atoms of three histidines--numbered 94, 96 and 119 (colored in yellow)--directly coordinate the zinc. These amino acids are highly conserved in all isozymes. Atoms from threonine 199 and glutamate 106 (colored magenta) interact indirectly through the bound water, shown with a red sphere. Note that these residues in addition to the histidine 64 (also colored in magenta) help to charge the zinc with a hydroxyl ion. Some of the isozymes have differences in these and other residues, which may explain their difference in enzyme activity.Zinc is the key to this enzyme reaction. The water bound to the zinc ion is actually broken down to a proton and hydroxyl ion. Since zinc is a positively charged ion, it stabilizes the negatively charged hydroxyl ion so that it is ready to attack the carbon dioxide. A close up of the amino acid side chains in the active site and the zinc ion is shown in the two right hand figures. The top figure shows a hydroxyl ion, shown with a red sphere, bound to the zinc ion in PDB entry 1ca2. Zinc directs the transfer of this bound hydroxyl to carbon dioxide, forming a bicarbonate ion. The bottom figure shows an intermediate structure where the bicarbonate ion, shown with red and white spheres, has just formed and is still bound to the enzyme (PDB entry 1cam). Note that the side chain for amino acid 199 is modeled as an alanine in this structure. Histidine 64 swings towards and away from the zinc ion in each cycle of enzyme action while helping the zinc to recharge with a new hydroxyl ion. The two positions of this residue, shown in the bottom right figure, represent its movement during enzyme action.
As soon as the zinc is reloaded with a new water molecule and the bicarbonate ion has been released, the enzyme will be ready for action on another carbon dioxide molecule.
These pictures were created with RasMol. You can create similar pictures by clicking on the accession codes above, and then picking one of the options under View Structure.
A list of all carbonic anhydrases in the PDB as of January, 2004 is available here. For more information on carbonic anhydrase, click here.
Next: Interaktive 3D-Animation
Previous: Carbonic Anhydrase in Health and Disease
Last changed by: A.Honegger,