|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
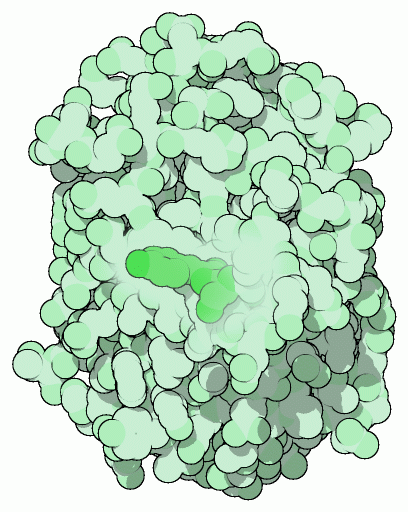
Green Fluorescent Protein (GFP)
Green Fluorescent Protein
The green fluorescent protein, shown here from PDB entry 1gfl, is found in a jellyfish that lives in the cold waters of the north Pacific. The jellyfish contains a bioluminescent protein-- aequorin--that emits blue light. The green fluorescent protein converts this light to green light, which is what we actually see when the jellyfish lights up. Solutions of purified GFP look yellow under typical room lights, but when taken outdoors in sunlight, they glow with a bright green color. The protein absorbs ultraviolet light from the sunlight, and then emits it as lower-energy green light.
So What?
You might be saying: who cares about this obscure little green protein from a jellyfish? It turns out that GFP is amazingly useful in scientific research, because it allows us to look directly into the inner workings of cells. It is easy to find out where GFP is at any given time: you just have to shine ultraviolet light, and any GFP will glow bright green. So here is the trick: you attach the GFP to any object that you are interested in watching. For instance, you can attach it to a virus. Then, as the virus spreads through the host, you can watch the spread by following the green glow. Or, you can attach it to a protein, and watch through the microscope as it moves around inside cells.Ready-Made
GFP is a ready-made fluorescent protein, so it is particularly easy to use. Most proteins that deal with light use exotic molecules to capture and release photons. For instance, the opsins in our eyes use retinol to sense light (see the Molecule of the Month on bacteriorhodopsin). These "chromophores" must be built specifically for the task, and carefully incorporated into the proteins. GFP, on the other hand, has all of its own light handling machinery built in, constructed using only amino acids. It has a special sequence of three amino acids: serine-tyrosine-glycine (sometimes, the serine is replaced by the similar threonine). When the protein chain folds, this short segment is buried deep inside the protein. Then, several chemical transformations occur: the glycine forms a chemical bond with the serine, forming a new closed ring, which then spontaneously dehydrates. Finally, over the course of an hour or so, oxygen from the surrounding environment attacks a bond in the tyrosine, forming a new double bond and creating the fluorescent chromophore. Since GFP makes its own chromophore, it is perfect for genetic engineering. You don't have to worry about manipulating any strange chromophores; you simply engineer the cell with the genetic instructions for building the GFP protein, and GFP folds up by itself and starts to glow.Engineering GFP
The uses of GFP are also expanding into the world of art and commerce. Artist Eduardo Kac has created a fluorescent green rabbit by engineering GFP into its cells. Breeders are exploring GFP as a way to create unique fluorescent plants and fishes. GFP has been added to rats, mice, frogs, flies, worms, and countless other living things. Of course, these engineered plants and animals are still controversial, and are spurring important dialogue on the safety and morality of genetic engineering.Next: Improving GFP
Last changed by: A.Honegger,