|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Hemoglobin
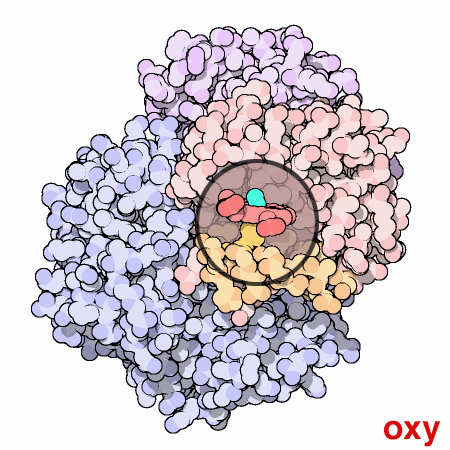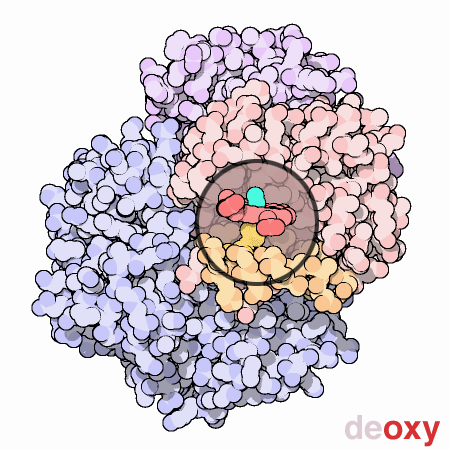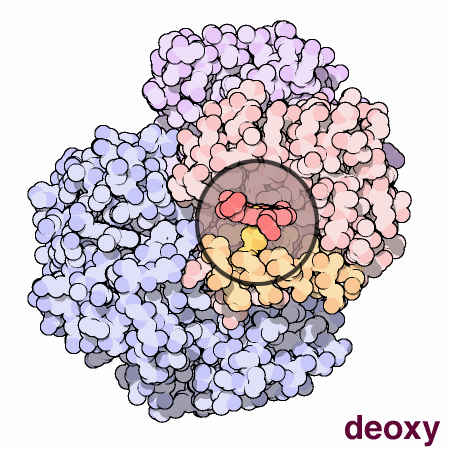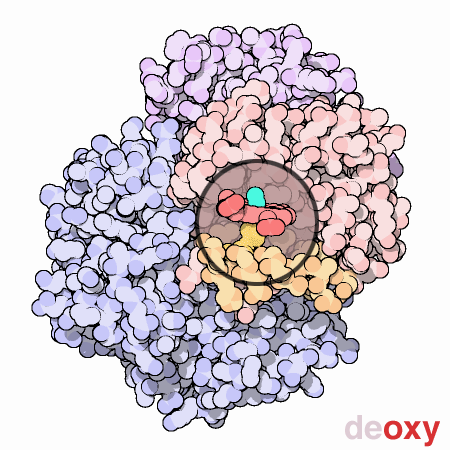
Cooperation Makes It Easier
Hemoglobin is a remarkable molecular machine that uses motion and small structural changes to regulate its action. Oxygen binding at the four heme sites in hemoglobin does not happen simultaneously. Once the first heme binds oxygen, it introduces small changes in the structure of the corresponding protein chain. These changes nudge the neighboring chains into a different shape, making them bind oxygen more easily. Thus, it is difficult to add the first oxygen molecule, but binding the second, third and fourth oxygen molecules gets progressively easier and easier. This provides a great advantage in hemoglobin function. When blood is in the lungs, where oxygen is plentiful, oxygen easily binds to the first subunit and then quickly fills up the remaining ones. Then, as blood circulates through the body, the oxygen level drops while that of carbon dioxide increases. In this environment, hemoglobin releases its bound oxygen. As soon as the first oxygen molecule drops off, the protein starts changing its shape. This prompts the remaining three oxygens to be quickly released. In this way, hemoglobin picks up the largest possible load of oxygen in the lungs, and delivers all of it where and when needed.In this animated figure, the heme group of one subunit, shown in the little circular window, is kept in one place so that you can see how the protein moves around it when oxygen binds. The oxygen molecule is shown in blue green. As it binds to the iron atom in the center of the heme, it pulls a histidine amino acid upwards on the bottom side of the heme. This shifts the position of an entire alpha helix, shown here in orange below the heme. This motion is propagated throughout the protein chain and on to the other chains, ultimately causing the large rocking motion of the two subunits shown in blue. The two structures shown are PDB entries 2hhb and 1hho.
Next: Troubled Hemoglobins
Previous: Hemoglobin
Last changed by: A.Honegger,