|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Ferritin and Transferrin
Iron is found everywhere on the Earth, so it is no surprise that living cells use iron ions in many ways. We use iron throughout our body, for many tasks. Iron ions bind strongly and specifically to small molecules such as oxygen, making it an essential tool for manipulating these elusive molecules. Iron ions also cycle easily between the ferrous and ferric forms, providing a handy tool for manipulating individual electrons. Iron ions, however, pose a great challenge in our modern biological environment. The water filling cells and the oxygen in the air together conspire to convert iron ions to the ferric state, which is highly insoluble, forming rust-like oxides. The cell must somehow shelter iron ions so that they may be stored and delivered in the necessary quantities. This is the job of ferritin and transferrin.
Iron Storage
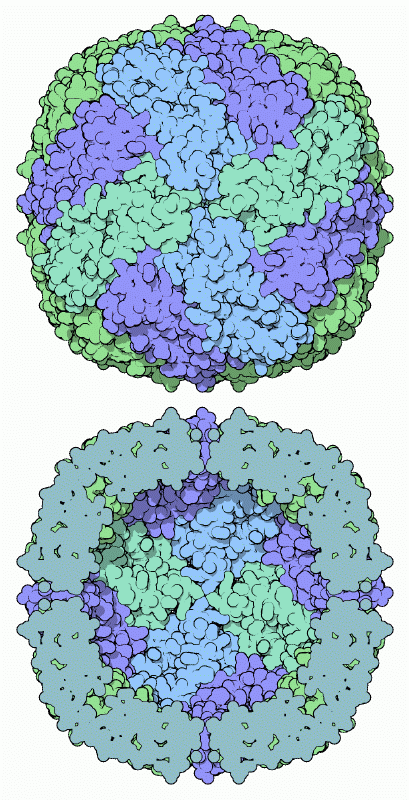
Inside cells, extra iron ions are locked safely in the protein shell of ferritin, shown here from PDB entry 1fha. Ferritin is composed of 24 identical protein subunits that form a hollow shell. The bottom illustration shows the hollow shell cut in half, showing the chamber inside and a few of the pores that lead inside the shell. After entering the ferritin shell, iron ions are converted into the ferric state, where they form small crystallites along with phosphate and hydroxide ions. There is room to pack about 4500 iron ions inside.Rich in Iron
We have about 3.7 grams of iron in our body, painstakingly gathered from iron in our diet. About 2.5 grams are locked inside the hemoglobin in our blood, where they assist in the transport of oxygen. This is a valuable and essential resource, so special mechanisms for the recycling of this iron have been developed. Another few tenths of a gram are found in myoglobin, which also assists in oxygen management. A remarkably small amount--about 0.02 g--is distributed between the many different proteins that transfer electrons, such as the proteins of the electron transport chain that create most of our cellular ATP supplies. The rest, about a gram, is stored inside ferritin to fulfill future needs.Next: Transporting Iron Ions
Last changed by: A.Honegger,