|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Dihydrofolate Reductase
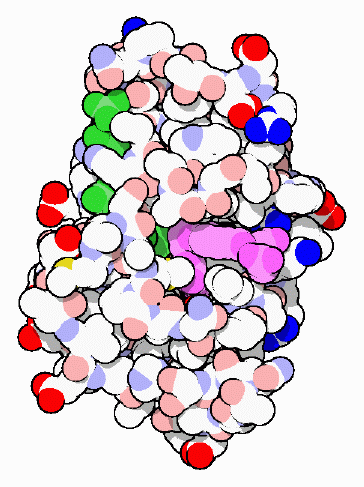
Dihydrofolate reductase is a small enzyme that plays a supporting role, but an essential role, in the building of DNA and other processes. It manages the state of folate, a snaky organic molecule that shuttles carbon atoms to enzymes that need them in their reactions. Of particular importance, the enzyme thymidylate synthase uses these carbon atoms to build thymine bases, an essential component of DNA. After folate has released its carbon atoms, it has to be recycled. This is the job performed by dihydrofolate reductase.
A Protein Jig
Dihydrofolate reductase, shown here from PDB entry 7dfr, juggles two relatively large molecules in its reaction. It has a long groove that binds to folate at one end, shown here in purple, and to NADPH at the other end, shown here in green. As you can see, the protein wraps sidechains around the two molecules, positioning them tightly next to one another. Then, the enzyme transfers hydrogen atoms from NADPH to the folate, converting folate to a useful reduced form.A Target in the Fight Against Cancer
Enzymes with essential roles are sensitive targets for drug therapy. Dihydrofolate reductase was the first enzyme to be targeted for cancer chemotherapy. The first drug used for cancer chemotherapy was aminopterin. It binds to dihydrofolate reductase a thousand times more tightly than folate, blocking the action of the enzyme. Today, methotrexate and other variations on aminopterin are used, because of their tighter binding and better clinical characteristics. Since these drugs attack a key step in the production of DNA, they tend to kill cells that are actively growing rather than cells that are not growing. Since cancer cells are often the most rapidly reproducing cells in a patient, the drug will have the strongest effect on the cancer cells. The side effects of chemotherapy, however, are the result of the drug on other normally-growing tissues, such as hair follicles and the lining of the stomach.Last changed by: A.Honegger,