|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Nitrogenase
Nitrogen is needed by all living things to build proteins and nucleic acids. Nitrogen gas is very common on the earth, as it comprises just over 75% of the molecules in air. Nitrogen gas, however, is very stable and difficult to break apart into individual nitrogen atoms. Usable nitrogen, in the form of ammonia or nitrate salts, is scarce. Often, the growth of plants is limited by the amount of nitrogen available in the soil. Small amounts of usable forms of nitrogen are formed by lightning and the ultraviolet light from the sun. Significant amounts of nitrogen are fed to plants in the form of industrial fertilizers. But the lion's share of usable nitrogen is created by bacteria, using the enzyme nitrogenase.
Fixing Nitrogen
Nitrogen-fixing bacteria have the ability to convert nitrogen gas into ammonia, which is easily combined with other raw materials to form the building blocks of proteins and nucleic acids. This process requires extreme measures, because nitrogen gas is so stable. The industrial process used to create ammonia requires high temperatures and pressures of 300 atmospheres, along with catalysts. In nitrogen-fixing bacteria, the enzyme nitrogenase drives the reaction with a large quantity of ATP, and uses a collection of metal ions, including an unusual molybdenum ion, to perform the reaction.An Expensive Reaction
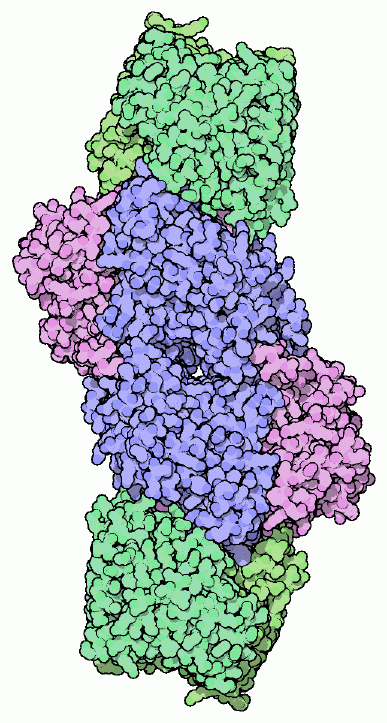
Nitrogenase is composed of two components, shown here from entry 1n2c. The MoFe protein, shown in blue and purple, contains all of the machinery to perform the reaction, but requires a steady source of electrons. The reaction requires the addition of six electrons for each nitrogen molecule that is split into two ammonia molecules. The Fe protein, shown in green, uses the breakage of ATP to pump these electrons into the MoFe protein. In the typical reaction, two molecules of ATP are consumed for each electron transferred. Nitrogenase also converts hydrogen ions to hydrogen gas at the same time (this might be an obligatory part of the nitrogen-splitting reaction, or it might be a simple side effect), thus consuming even more ATP in the process.This is a large investment in energy, but well worth the effort if nitrogen is not available in the environment. Fortunately, nitrogen-fixing bacteria are found throughout the world, and are often found in partnerships with plants. For instance, legumes build special nodules in their roots that provide a perfect home for the bacteria. The plants provide shelter and even a few essential nutrients, jealously guarding their guests, and the bacteria provide a steady supply of nitrogen.
Last changed by: A.Honegger,