|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
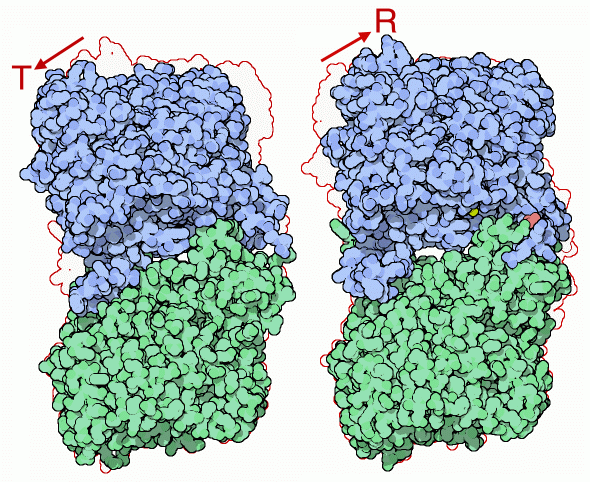
Glycogen Phosphorylase
Shape-shifting
Glycogen phosphorylase is activated by a change of shape. The structure on the left (PDB entry 8gpb) is in the inactive T state and the structure on the right (PDB entry 1gpa) is in the active R state. Compared with the pictures on the previous page, we are looking from the side and the active sites are on the left-hand side. (T stands for tense and R for relaxed, a notation developed when the first allosteric enzymes were being studied, although structures such as these have shown that the idea of tension does not really apply at the molecular level). The shift between the two shapes is controlled by phosphorylation of serine 14 or binding of AMP to the regulatory site. The R-state structure shown here has phosphates attached to the serines (colored pink) and a sulfate group in the site that binds to AMP (colored yellow).Next: Exploring the Structure
Previous: Glycogen Phosphorylase
Last changed by: A.Honegger,