|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Actin
Molecular Infrastructure
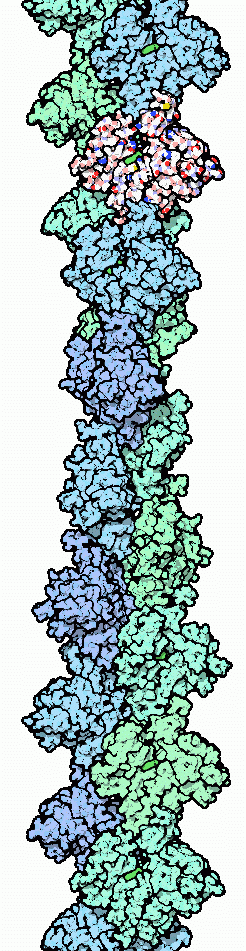
The complex ultrastructure of cells--their shape and internal structure--and the many motions of cells are largely supported by filaments of actin. A tangle of cross-linked actin filaments fills the cytoplasm of animal, plant and fungal cells, forming a "cytoskeleton" that gives the cell shape and form and provides a scaffold for organization. Tightly bundled actin filaments provide a sturdy backbone to extrude structures from the cell surface, such as the pseudopods used by amoebas for crawling and the finger-like microvilli of intestinal cells, which extend into the digestive tract and absorb nutrients. As we saw last month, actin also forms the ladder on which myosin climbs, providing the infrastructure for muscle contraction and creating the motion that we experience in our daily lives. Actin is plentiful throughout the body as it performs these basic structural tasks: it may comprise 5 percent of the protein in a typical cell, or up to one fifth of the protein in special cases, such as muscle cells.A Dynamic Molecule
Actin has a rare combination of strength and sensitivity. Actin filaments are used in many of the most strenuous structural tasks, but at the same time, actin filaments are easily and continually disassembled. One of the great hallmarks of actin is its dynamic character. Actin filaments are continually built and broken down as the needs of the cell change from moment to moment. In special cases, such as muscle actin or the actin bundles in microvilli, a collection of specialized actin-binding proteins stabilize the filament, forming a more permanent structure. But the bulk of actin in typical cells is in constant flux, constantly forming filaments and breaking down for each new task.The dynamic character of actin is controlled by a molecule of ATP bound to each actin monomer. The state of this ATP determines the stability of the actin filament. Free actin typically holds an ATP molecule and binds tightly to growing filaments. After attaching, the ATP is broken and the actin subtly changes shape. This new form, with ADP bound, is not as stable in the filament and dissociates more easily. One of the unusual consequences of this behavior is "treadmilling." An actin filament will be continually building at one end, where new actin-ATP complexes are forming strong new connections, and at the same time slowly falling apart at the opposite end, where the actin-ADP form has weakened connections. Imagine the filament growing at one end and dissolving at the other, so that the whole structure slowly steps through the cell but never gets any longer or shorter.
Next: Controlled Growth
Last changed by: A.Honegger,