|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Pepsin
Acid Proteases
Pepsin is one example of a group of enzymes termed "acid proteases." In the case of pepsin, this name is doubly appropriate. Pepsin works its best in strong hydrochloric acid. But the similarity with the other enzymes pictured here refers to a second type of acid. The active site of the acid proteases rely on two acidic aspartate amino acids, which activate a water molecule and use it to cleave protein chains. These aspartates are pictured on the next page.
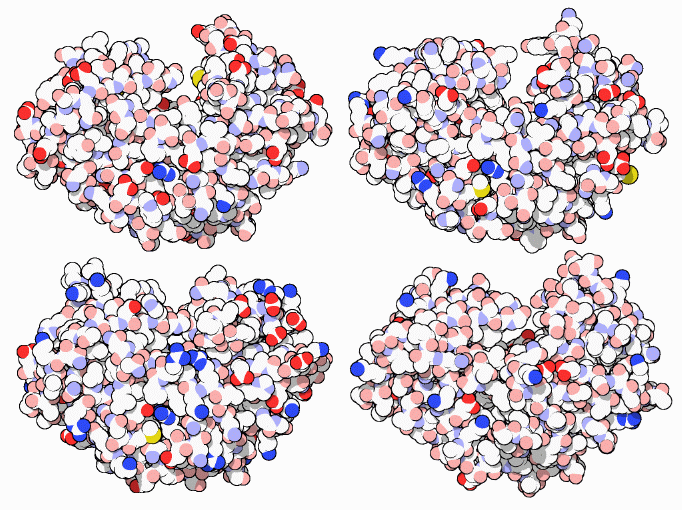
The acid proteases have evolved to fill several functional roles in different organisms. Pepsin, shown at upper left (PDB entry 5pep), is optimized for digestion of food in the acidic environment of the stomach. It is very promiscuous, cleaving proteins in many different places. Chymosin, shown at upper right (PDB entry 4cms), is made by young calves to break down milk proteins. A purified form of chymosin, taken from calf stomach, has been used for centuries to curdle milk in the production of cheese. Cathepsin D, shown at lower left (PDB entry 1lyb), digests proteins inside lysozomes, the tiny stomachs inside cells. Other cellular acid proteases, such as renin (not shown, PDB entry 1hrn), are designed to make very specific cuts in one particular protein, aiding in the maturation of a hormone or structural protein. Endothiapepsin, shown at lower right (PDB entry 4ape), is made by a fungus and excreted into the surrounding environment, breaking up the surrounding proteins and allowing the fungus to feed on the pieces.
Next: Exploring the Structure
Previous: Pepsin
Last changed by: A.Honegger,