|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Cytochrome C Oxydase
Evolution of an Enzyme
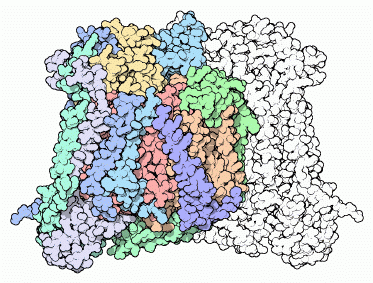
Oxygen is consumed in special compartments inside our cells, termed mitochondria. When looking in the electron microscope, mitochondria bear an uncanny resemblance to simple bacteria. Today, many biologists believe that mitochondria are actually the result of a bacterial invasion sometime in the distant past. A bacterium squeezed inside another cell, but didn't kill the cell in the process. Instead they lived peacefully together. When the cell divided, the bacteria inside did too, so both daughter cells had bacterial invaders as well. Over many generations, the two became totally dependent on one another. The bacteria inside specialized on energy production and the cell provided protection and nutrients. Today, these bacteria are our mitochondria.Evidence of this symbiotic relationship is found in cytochrome c oxidase. The enzyme from mammals is very complex, composed of 13 separate protein chains (the cow enzyme is shown here, from PDB entry 1oco). Three large chains at the core of the enzyme, colored yellow, orange and red here, perform most of the work. Around this are ten smaller chains, colored in greens and blues.
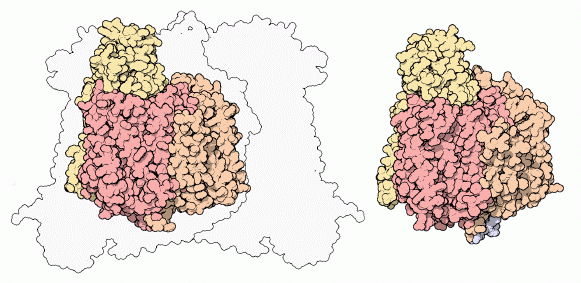
If we look at the cytochrome c oxidase made by a bacterium, PDB entry
1qle shown here on the right, it is much simpler.
It is composed of only four
chains. Three are similar to the core chains in our enzyme, colored yellow,
orange, and red. One additional small chain can just be seen poking out the
bottom here, in blue. Notice how similar this enzyme is to the core of the
mammalian enzyme, shown on the left.
This similarity is compelling, but the story is even more interesting. Our
mitochondria actually contain all of the machinery needed to build their
own proteins--they have DNA, ribosomes, and everything. In our cells, the
three core subunits of cytochrome c oxidase are built inside our
mitochondria, but the remaining ten small chains are built outside in the
cytoplasm and then added to the mitochondria later. So, our mitochondria
build a bacteria-like enzyme, which our cells then decorate with other
proteins to customize its function.
Next: Interaktive 3D-Animation
Previous: Exploring the Structure
Last changed by: A.Honegger,