|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Cytochrome C Oxydase
Oxygen and Life
Oxygen is an unstable molecule. If given a chance, it will break apart and combine with other molecules. This is the process of oxidation, seen in our familiar world as the rusting of iron in cars and nails. But, surprisingly, the unusual electronic properties of oxygen molecules make this reaction very slow. So, paper doesn't spontaneously burn up--flames must be kindled.All animals and plants, and many microorganisms, use the instability of oxygen to power the processes of life. The molecules in food are oxidized and the energy is used to build new molecules, to swim or crawl, and to reproduce. Food is not oxidized in a fiery flame, however. It is oxidized in many slow steps, each carefully controlled and designed to capture as much useable energy as possible.
Cytochrome c oxidase controls the last step of food oxidation. At this point, the atoms themselves have all been removed and all that is left are a few of the electrons from the food molecules. Cytochrome c oxidase, shown here, takes these electrons and attaches them to an oxygen molecule. Then, a few hydrogen ions are added as well, forming two water molecules.
Charging the Battery
The reaction of oxygen and hydrogen to form water is a favorable process, releasing a good deal of energy. In our familiar world, hydrogen and oxygen combine explosively, which is the reason that dirigibles are filled with helium instead of hydrogen. In our cells, however, the energy is carefully harnessed by cytochrome c oxidase to charge a battery, or perhaps more correctly, to charge a capacitor.
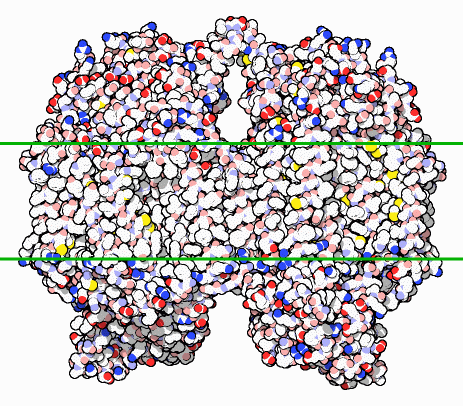
Cytochrome c oxidase is a membrane protein. In the picture, notice the region between the green lines. Most of the surface atoms there are carbon (white) and sulfur (yellow). In the cell, these atoms are buried inside a membrane. Notice that the regions at the top and bottom are covered with charged oxygen and nitrogen atoms, colored bright red and blue here. These regions, which prefer a watery environment, stick out on opposite faces of the membrane. This arrangement is perfect for the job performed by cytochrome c oxidase, which uses the reaction of oxygen to water to power a molecular pump. As oxygen is consumed, the energy is stored by pumping hydrogen ions from one side of the membrane to the other. Later, the energy can be used to build ATP or power a motor by letting the hydrogen ions seep back across the membrane.
Next: Exploring the Structure
Last changed by: A.Honegger,