|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Trypsin
Exploring the Structure
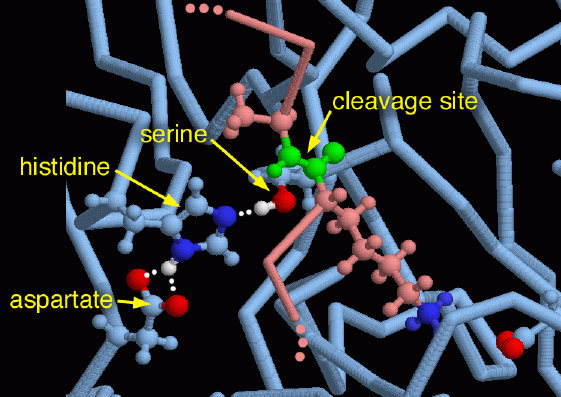
As you look through the PDB, you will find many other examples of serine proteases, built for digestion, hormone activation, blood clotting, immune system activation, and many other functions. They share an unusual collection of amino acids designed to assist protein-cutting reactions, which have been discovered again and again by evolution. The center of the machinery is a serine amino acid that is activated by a histidine and an aspartate. Together, these three amino acids have been termed the charge relay system. The histidine and the aspartate assist in the removal of the hydrogen atom from the serine (colored white), which makes it more reactive when attacking the target protein chain. This illustration was created using PDB entry 2ptc, which has an inhibitor protein (colored pink) bound in the active site. The site of cleavage in this inhibitor, colored green here, is held just far enough away that it is not cleaved the way most proteins would be in this location. Notice also the long lysine amino acid extending down to the lower right from the cleavage site, where it interacts with another aspartate in the enzyme (shown down in the lower right corner with red oxygens). Through this interaction, trypsin favors cutting at places next to lysine or arginine amino acids.This illustration was created with RasMol. You can create similar illustrations by clicking on the accession codes in this Molecule of the Month, and picking one of the options under View Structure.
A list of all trypsin structures in the PDB as of October, 2003, is available here. For more information on trypsin and other serine proteases, click here. To explore trypsin from a genomic perspective, take a look at the Protein of the Month feature at the European Bioinformatics Institute.
Next: Interaktive 3D-Animation
Previous: The Perils of Proteases
Last changed by: A.Honegger,