|
Inhaltsübersicht | Nanomaschinen | Moleküle | Programme | Kurse | Fun | Links |
||
| > |
Multidrug Resistance Transporters
Exploring the Structure
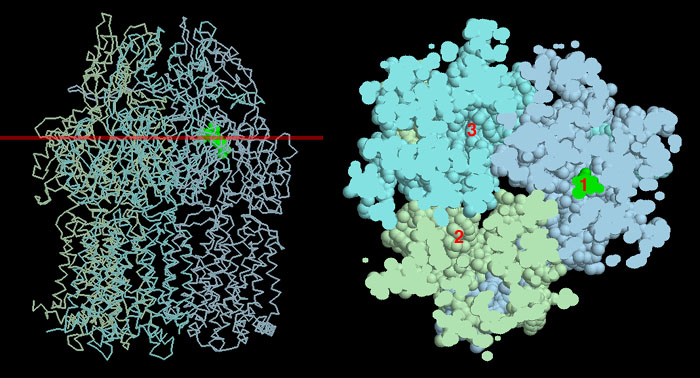
The AcrB transporter is currently thought to use an unusual cyclic mechanism to pump drugs out of the cell. The protein is composed of three identical subunits, and in recent crystal structures, the three subunits have slightly different conformations. The structure shown here, from PDB entry 2drd, has the antibiotic minocycline bound in one subunit. The picture on the left shows a backbone diagram of the whole protein, with the drug in green. If you cut away the bottom half of the protein at the red line, and then look at the slice that is left, you get the picture on the right. It shows all three binding sites. The first site (marked 1) has the drug bound, shown in green. The second side (marked 2) has shifted to a new conformation that has opened pathways to the pore at the middle of the complex. The final site (marked 3) is in a third conformation that connects to the membrane (not seen in this picture) and can thus bind to new drug molecules. By cycling through these three conformations, the transporter pulls drugs out of the membrane and then pushes them outside.
You can also take a look at several earlier structures of AcrB (such as PDB entries 1oy8 or 1oyd) that have drugs bound in a different place, in a central pore formed by all three subunits. These structures might show a different mechanism, where drugs are taken out of the cytoplasm and then pumped across the membrane.
These pictures were created with RasMol. You can make similar pictures by clicking on the accession codes here and choosing one of the options under Images and Visualization. To see the scientific papers that I used for this article, click here. Also available are related entries in the PDB as determined by a keyword search on October 30, 2007 for 'multidrug transporter'.
Previous: Transporters in All Shapes and Sizes
Last changed by: A.Honegger,