|
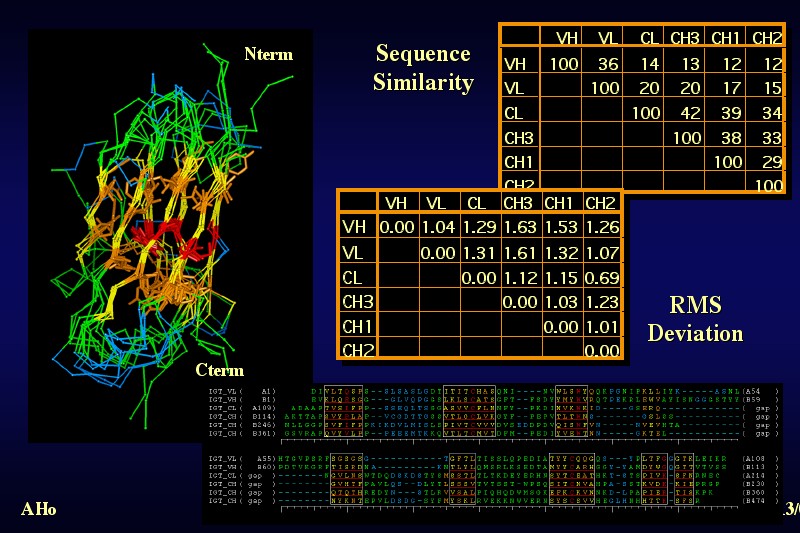
The six different immunoglobulin domains of Igg2a have been superimposed by a least-square fit of the core C-alpha positions indicated in yellow, orange and red Although the domains show very limited sequence similarity: 12% is usually not sufficient to recognize the relationship from sequence data alone, and the sequence alignment can only be gapped properly by looking at the structure and comparing the sequence alignment to the positional equivalence of residues, the folding topology of all six domains is the same, and the structural similarity of the core is striking. The most conserved features are a disulfide bond linking the two sheets and a core tryptophane residue. The rest of the core is packed with various aliphatic and aromatic residues. |